Share this
SARS-CoV-2 antibodies: 3 classes of tests...which is most useful?
by James Rudge, PhD, Technical Director, Trajan on Jun 5, 2020 9:15:00 AM
The scientific and medical communities continue investigating potential vaccines and therapies against SARS-CoV-2, or severe acute respiratory syndrome coronavirus 2, the novel coronavirus that causes COVID-19 illness. Many believe that testing for the presence of SARS-CoV-2 raised antibodies is one of the best ways to gain an understanding of a possible full or partial immunity to the disease. Furthermore antibody testing will impart an understanding of which interventions will be most effective in preventing and treating these infections.

Throughout the Spring of 2020, we have received equal amounts of insightful information and misinformation about SARS-CoV-2 antibody testing. Conflicting news reports about exactly what antibody testing can—and can’t—tell us has led to widespread frustration and confusion.
Some serology studies have been launched in an effort to track the presence of antibodies in blood samples from people who have already been exposed to the coronavirus or recovered from a COVID-19 infection. Will these “serology surveys” provide us with definitive information about herd immunity and insights on how long immunity might last? We’re not yet sure.
Taking a Closer Look at SARS-CoV-2 Antibody Testing
To understand why antibody testing doesn’t always lead to definitive answers about a disease, or about immunity to that disease, let’s take a closer look at how antibody testing works.
When we talk about testing for antibodies of SARS-CoV-2, we are actually talking about three classes of antibodies: IgA, M and G. Among scientists at the forefront of COVID-19 research, it seems that M and G are the most popular classes for testing right now.
Currently, there are tests, either recently launched or in the process of being developed, that can detect antibodies A,M and G, or M & G, or just G. However, it is important to note that all SARS-CoV-2 antibody tests are not going to be as good as one another. Some will be more sensitive, some will be more specific, and some will be both with little to no false positives or negatives. What does this mean? To illustrate, let’s examine an ELISA test, or enzyme-linked immunoassay. An ELISA is a laboratory test that detects antibodies in blood. In the case of SARS-CoV-2, the antibodies which are being are investigated are being raised against the spike protein and, in some cases, the nucleocapsid.
A lab that is conducting SARS-CoV-2 antibody testing might use an ELISA test, which simultaneously looks for M and G, but can’t distinguish between the two. The test simply indicates that a person has been exposed to the coronavirus and has developed some kind of immune response. It does not say if the individual has developed IgG antibodies that can possibly lead to an individual developing an immunity, which has been proven to occur for other viral infections. Moreover, there are ELISAs that look for one type of antibody, usually IgG, which indeed tells us if a person has developed possible immunity.
There are also multiplex assays that have the potential to look for all three classes of antibodies, and even various mutations of the spike protein (the main immunoassay target) of the novel coronavirus. However, it is worth noting that the processes and methods involved can vary widely—even the lateral flow devices differ. There are some that can measure both M and G. Among all these techniques, IgG is far and away the most popular.
Many labs are now optimized for antibody testing using remote blood samples collected with the Mitra® device, which is based on volumetric absorptive microsampling, or VAMS® technology. The VAMS technology allows people to collect a capillary blood sample in the safety of their homes and the dried sample is then sent a laboratory in the regular post. Moreover, the VAMS samples can be measured on the above-mentioned immunoassay platforms regardless of which class of immunoglobulins are being measured. Scientists are still refining their testing methods. Some assays will give more false positives or negatives than others, which will define the intended market for the test.
For additional information on immunoassays and extraction methods using Mitra and VAMS for SARS-CoV-2 testing, please see our “Tech Brief: A Review of Extraction Methods Using Mitra from the Current Literature.”
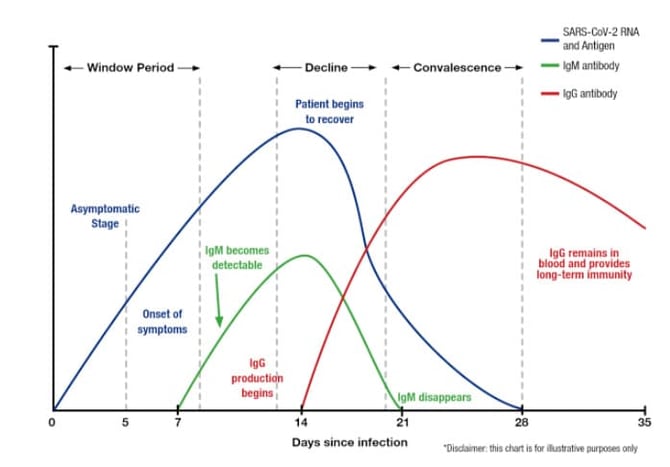
This chart below, which appeared in an article titled “Why Do We Need Antibody Tests for COVID-19 and How to Interpret Test Results” from Diazyme Laboratories, is for illustrative purposes only. It shows variation of levels of SARS-CoV-2 RNA and Antigen IgM and IgG after infection.
Sources:
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7184973/
https://thenativeantigencompany.com/why-we-need-antigen-and-antibody-tests-for-covid-19/
For information on microsampling for SARS-CoV-2, visit our infectious disease resources.

Share this
- Microsampling (206)
- Research, Remote Research (119)
- Venipuncture Alternative (105)
- Clinical Trials, Clinical Research (83)
- Mitra® Device (73)
- Therapeutic Drug Monitoring, TDM (51)
- Dried Blood Spot, DBS (39)
- Biomonitoring, Health, Wellness (30)
- Infectious Disease, Vaccines, COVID-19 (24)
- Blood Microsampling, Serology (23)
- Omics, Multi-Omics (21)
- Decentralized Clinical Trial (DCT) (20)
- Specimen Collection (18)
- Toxicology, Doping, Drug/Alcohol Monitoring, PEth (17)
- Skin Microsampling, Microbiopsy (14)
- hemaPEN® Device (13)
- Preclinical Research, Animal Studies (12)
- Pharmaceuticals, Drug Development (9)
- Harpera Device (7)
- Industry News, Microsampling News (5)
- Antibodies, MAbs (3)
- Company Press Release, Product Press Release (3)
- Environmental Toxins, Exposures (1)
- July 2025 (1)
- May 2025 (1)
- April 2025 (2)
- December 2024 (2)
- November 2024 (1)
- October 2024 (3)
- September 2024 (1)
- June 2024 (1)
- May 2024 (1)
- April 2024 (4)
- March 2024 (1)
- February 2024 (2)
- January 2024 (4)
- December 2023 (3)
- November 2023 (3)
- October 2023 (3)
- September 2023 (3)
- July 2023 (3)
- June 2023 (2)
- April 2023 (2)
- March 2023 (2)
- February 2023 (2)
- January 2023 (3)
- December 2022 (2)
- November 2022 (3)
- October 2022 (4)
- September 2022 (3)
- August 2022 (5)
- July 2022 (2)
- June 2022 (2)
- May 2022 (4)
- April 2022 (3)
- March 2022 (3)
- February 2022 (4)
- January 2022 (5)
- December 2021 (3)
- November 2021 (5)
- October 2021 (3)
- September 2021 (3)
- August 2021 (4)
- July 2021 (4)
- June 2021 (4)
- May 2021 (4)
- April 2021 (3)
- March 2021 (5)
- February 2021 (4)
- January 2021 (4)
- December 2020 (3)
- November 2020 (5)
- October 2020 (4)
- September 2020 (3)
- August 2020 (3)
- July 2020 (6)
- June 2020 (4)
- May 2020 (4)
- April 2020 (3)
- March 2020 (6)
- February 2020 (3)
- January 2020 (4)
- December 2019 (5)
- November 2019 (4)
- October 2019 (2)
- September 2019 (4)
- August 2019 (4)
- July 2019 (3)
- June 2019 (7)
- May 2019 (6)
- April 2019 (5)
- March 2019 (6)
- February 2019 (5)
- January 2019 (8)
- December 2018 (3)
- November 2018 (4)
- October 2018 (7)
- September 2018 (6)
- August 2018 (5)
- July 2018 (8)
- June 2018 (6)
- May 2018 (5)
- April 2018 (6)
- March 2018 (4)
- February 2018 (6)
- January 2018 (4)
- December 2017 (2)
- November 2017 (3)
- October 2017 (2)
- September 2017 (4)
- August 2017 (2)
- July 2017 (4)
- June 2017 (5)
- May 2017 (6)
- April 2017 (6)
- March 2017 (5)
- February 2017 (4)
- January 2017 (1)
- July 2016 (3)
- May 2016 (1)
- April 2016 (2)


Comments (1)