Mitra® VAMS® Microsampling Collection Video Now Available in Spanish and Arabic


Mitra® VAMS® Microsampling Collection Video Now Available in Spanish and Arabic
February 3,2026
1
min read
Success with Microsampling in Clinical Trials Live Discussion


Success with Microsampling in Clinical Trials Live Discussion
January 22,2026
1
min read
Trajan appoints AppMed Inc. as Canadian distributor for Microsampling technologies, featuring Mitra® and Harpera®


Trajan appoints AppMed Inc. as Canadian distributor for Microsampling technologies, featuring Mitra® and Harpera®
December 3,2025
3
min read
Neoteryx and Cellecta partner to introduce innovative finger-prick blood test for comprehensive whole-genome expression profiling
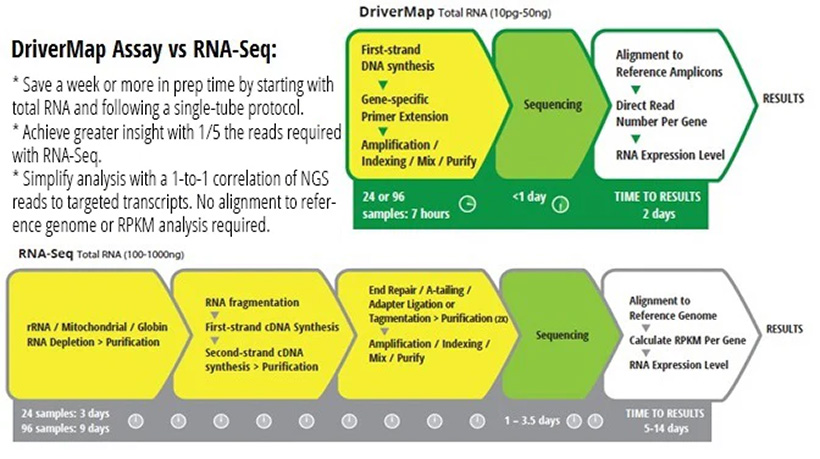

Neoteryx and Cellecta partner to introduce innovative finger-prick blood test for comprehensive whole-genome expression profiling
May 14,2025
2
min read
Neoteryx EU authorized representative update

Neoteryx EU authorized representative update
April 10,2025
1
min read
Revolutionizing PFAS Detection with Mitra VAMS Technology


Revolutionizing PFAS Detection with Mitra VAMS Technology
February 27,2025
1
min read
