how Microbiopsy is helping to better understand leishmania major infection
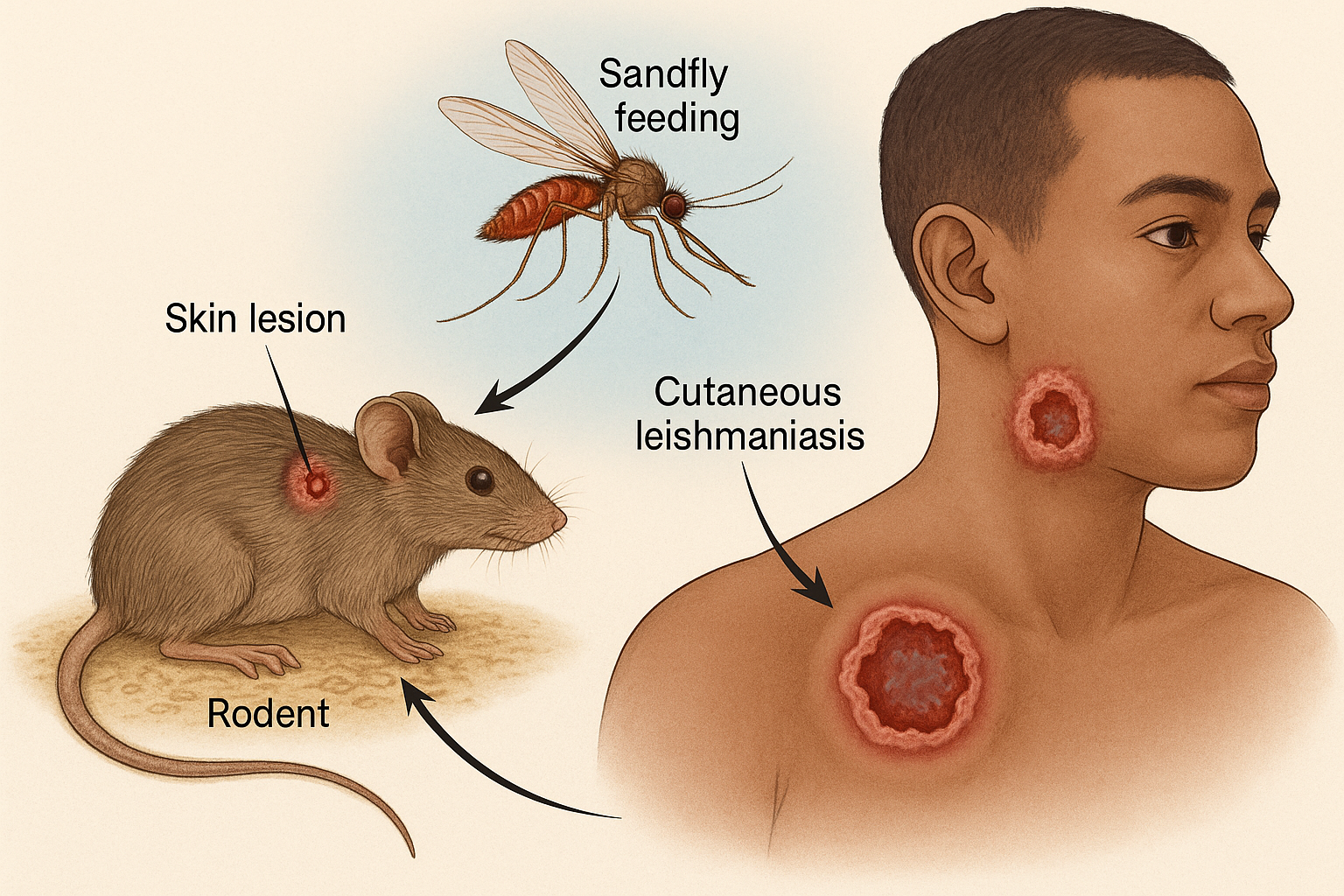

how Microbiopsy is helping to better understand leishmania major infection
Jul 11, 2025 11:52:19 AM
6
min read
Mitra® Microsampling: Advancing Pre-Clinical RNA Biomarker Research


Mitra® Microsampling: Advancing Pre-Clinical RNA Biomarker Research
May 14, 2025 4:40:21 PM
2
min read
wipe or not to wipe, that is the question!

wipe or not to wipe, that is the question!
Apr 24, 2025 9:59:52 AM
4
min read
exploring new therapies targeting inflammatory skin diseases with novel, minimally invasive skin microbiopsy

exploring new therapies targeting inflammatory skin diseases with novel, minimally invasive skin microbiopsy
Apr 9, 2025 10:59:08 AM
7
min read
the viability of Mitra devices for therapeutic drug monitoring

the viability of Mitra devices for therapeutic drug monitoring
Dec 16, 2024 1:32:11 PM
5
min read
remote dried blood sampling offers a solution to metabolite stabilization

remote dried blood sampling offers a solution to metabolite stabilization
Dec 13, 2024 10:38:38 AM
5
min read
