eliminate complex surgical processes
minimally invasive and virtually painless, even on cosmetic and sensitive areas
specimen suited for modern molecular analysis techniques
minimally invasive Harpera Microbiopsy Punch
The Harpera™ Microbiopsy™ Punch facilitates the collection of skin specimens using a minimally invasive method. The Harpera device is currently in development and available as an Investigational Use Only (IUO) device and its design is intended to offer a suture-free, and virtually painless option for scarless skin biopsy procedure. The small size and portability of the Harpera Microbiopsy Punch make it a user-friendly departure from conventional skin biopsy devices. Initial clinical performance studies indicate that no anesthesia or post-biopsy suturing is required during or after the Microbiopsy punch procedure. Biopsies taken with the Harpera device have been used in different testing methodologies, but they appear to be particularly suited for modern molecular techniques.
a scarless skin biopsy technique
The patented Microbiopsy technology is at the heart of the Harpera device, featuring a Microbiopsy collector crafted from high-precision laser-cut stainless steel. The design of the microbiopsy collector allows seamless and virtually painless penetration of the skin tissues to collect thousands of skin cells within its embodiment. The handle allows safe and easy manipulation during the sampling procedure and the retrieval of the skin specimen.
Simply available in one format, Harpera devices already meet diverse end-user and analytical needs to collect skin specimens for targeted biomarker analysis, making it a versatile choice for various dermatological research settings.
Minimally Invasive
The Microbiopsy design offers a virtually painless, suture-free experience, ideal for collecting specimens in sensitive areas and at multiple sampling sites.
Precision Sampling
Collects specimens within and at vicinity of targeted site, Suitable for all topical sampling procedures.
Ease of Use
Designed for facilitating the sampling procedure with a controlled punch. Safe manipulation of device before and after procedure.
Versatility
Suitable for a range of clinical and research dermatology applications, advancing the way of collecting skin specimens.
successful uses
Explore a collection of journal articles, interviews, and R&D presentations detailing various research studies using the Harpera Microbiopsy Punch as an alternative to traditional skin biopsy
Harpera Microbiopsy Punch (IUO)
/harpera-scarless-skin-biopsy-tool_v2.webp?width=731&height=488&name=harpera-scarless-skin-biopsy-tool_v2.webp)
The Harpera Microbiopsy Punch is transforming clinical dermatology research by providing a highly efficient and minimally invasive skin biopsy solution. Research and clinical teams will benefit from its key features, designed for precise Microbiopsy collection:
- A Microbiopsy collector crafted from precision laser-cut stainless steel
- A safety cap and insert for enhanced protection
- An ergonomic handle for effortless handling
- Consistent and controlled punch actuation for reliable sample collection.
Product Description: The Harpera Microbiopsy Punch is a single-use manual skin punch, a hand-held disposable device intended to achieve a controlled skin puncture in order to obtain a specimen. The device is supplied individually packaged and sterile through gamma irradiation.
Regulatory status: For investigational use only (IUO) in clinical studies.
Format: 1x Harpera device in a Tyvek pouch, Sterile
# device per pack: 20 units in a dispensing box
Pack content: Harpera Devices, 1x IFU
Features:
- Microbiopsy collector from high-precision laser-cut stainless steel
- Safety cap and insert for your protection
- Convenient handle for easy manipulation
- Controlled and consistent punch actuation
Harpera Instructions For Use
Opening Harpera & Retrieving Microbiopsy Collector (Option 1)
Retrieving Microbiopsy Collector w/ Tweezers (Option 2)
- Harpera Microbiopsy Punch Infographic
- App Note -Automated RNA Purification from Harpera™ Microbiopsy™ Punches
- App Note - Automated DNA Purification from Harpera™ Microbiopsy™ Punches
- App Note - Optimization of RNA Isolation Methods for RNASeq Analysis Using the Harpera Microbiopsy Device (Trajan / P&G )
- Harpera Microbiopsy Punch Brochure
- User Guide
- Customer Questionnaire: Download and check the boxes that best align with your experience. Email back to: neo.info@trajanscimed.com
skin cancer | research publication list
Banan, P., et al. (2013). "Effects of exvivo skin microbiopsy on histopathologic diagnosis in melanocytic skin lesions." JAMA Dermatol 149(9): 1107-1109.
Prow, T.W., et al. (2013). "The opportunity for microbiopsies for skin cancer." Future Oncol9 (9): 1241-1243
McClenahan, P., et al. (2014). "BRAFV600E mutation status of involuting and stable nevi in dabrafenib therapy with or without trametinib." JAMA Dermatol 150(10): 1079-1082
Tan, J.-M., et al.(2015). "BRAFWild-Type Melano main Situ Arising In a BRAFV600E Mutant Dysplastic Nevus." JAMA Dermatology 151(4).
Dermatology Research Centre, School of Medicine, The University of Queensland (March 2016), “Natural history and properties of naevi in advanced melanoma patients receiving treatment”, ACTRN 12616000272493 (ANZCTR)
Sobarun, P., et al. (2017). "Microbiopsy Biomarker Profiling in a Superficial Melanoma Resembling a Pigmented Basal Cell Carcinoma." JAMA Dermatol 153(4): 334-336.
Jain, M., et al. (2022). "Minimally invasive microbiopsy for genetic profiling of melanocyticlesions: A case series." J Am Acad Dermatol 87(4): 903-904.
skin disease/disorders | research publication list
Yamada, M., et al.(2020). "Microbiopsy-based minimally invasive skin sampling for molecular analysis is acceptable to Epidermolysis Bullosa Simplex patients where conventional diagnostic biopsy was refused." Skin Res Technol.
Preis, S., et al. (2022). "Munich atopy prediction study (MAPS): protocol for a prospective birth cohort addressing clinical and molecular risk factors for a topic dermatitis in early childhood." BMJ Open12 (9):e059256.
infectious skin disease/disorders | research publication list
Carter, E., et al. (2023). "A feasibility study of controlled human infection with intradermal Bacillus Calmette-Guerin (BCG) injection: Pilot BCG controlled human infection model." Wellcome Open Res 8: 424.
Tom, L.N., et al. (2016). "Skin microbiopsy for HPVDNA detection in cutaneous warts." J Eur Acad Dermatol Venereol 30(12): e216-e217.
Kirstein, O.D., et al. (2017). "Minimally invasive microbiopsies: a novel sampling method for identifying asymptomatic, potentially infectious carriers of Leishmania donovani." Int J Parasitol 47(10-11): 609-616.
Churiso,G.,et al. (2020). "Minimally Invasive Microbiopsies as an Improved Sampling Method for the Diagnosis of Cutaneous Leishmaniasis." Open Forum Infect Dis 7(9): ofaa364.
Cloots, K.,et al. (2021). "Assessing L. donovani Skin Parasite Load: A Proof of Concept Study of a Microbiopsy Device in an Indian Setting." Front Cell Infect Microbiol 11: 645121.
14. Cutaneous Leishmaniasis diagnostics: microbiopsy device for minimally invasive sampling - YouTube
Owen, S.I., et al. (2021). "Evaluation of qPCR on blood and skin microbiopsies, peripheral blood buffy coat smear, and urine antigen ELISA for diagnosis and test of cure for visceral leishmaniasis in HIV- coinfected patients in India: a prospective cohort study." BMJ Open 11(4): e042519.
Liverpool School of Tropical Medicine (April2023). “Using BCG Vaccine to Understand Tuberculosis Infection”, NCT05820594 (ClinicalTrials.gov)
Van Henten, S., et al. (2024)."Evaluation of Less Invasive Sampling Tools for the Diagnosis of Cutaneous Leishmaniasis." Open Forum Infect Dis 11(4): ofae113.
Duncan, N. R., et al. (2025). "From skin to sequence: pan-species detection and speciation of Leishmania." J Infect Dis.
Vojtkova, B., et al. (2025). "Infectiousness of Leishmania major to Phlebotomus papatasi: differences between natural reservoir host Meriones shawi and laboratory model BALB/c mice." PLoS Negl Trop Dis 19(6): e0013183.
general dermatology | research publication list
Primiero, C. A., et al. (2024). "Skin 2.0: How Cutaneous Digital Twins Could Reshape Dermatology." J Invest Dermatol.
Lin, L .L. and T.W. Prow (2017). "Novel microdevices for controlled blood and skin extraction, NHMRC." Impact 2017(6): 58-60.
Michele Fimiani, P.R., Elisa Cinotti (2020).Technology in Practical Dermatology, Springer Cham.
Hadeler E, Mosca M, Hong J, Brownstone N, Liao W, Bhutani T. "Innovations in translational research in dermatology: minimally invasive methods for bio-sample acquisition." Dermatol Online J. 2021 Oct15;27(10).
method development | research publication list
Lin, L.L.,et al. (2013). "Microbiopsy engineered for minimally invasive and suture-free sub-millimetre skin sampling." F1000Res 2: 120.
Lei,B.U.W.,et al.(2019)."Absorbent Microbiopsy Sampling and RNA Extraction for Minimally Invasive, Simultaneous Blood and Skin Analysis."JoVE (144): e58614.
cosmeceutical applications | research publication list
Yamada, M., et al. (2020). "A minimally invasive clinical model to test sunscreen toxicity based on oxidative stress levels using microbiopsy and confocal microscopy-A Proof of concept study." Int J Cosmet Sci.
Harpera news and blogs
Explore blogs that summarize how early adopters of the Harpera Microbiopsy Punch are performing non-surgical skin biopsies.
voices from the field: exploring Harpera’s global impact on dermatological research
Explore firsthand accounts from distinguished researchers as they share their groundbreaking work with the Harpera™ Microbiopsy™ Punch. These enlightening interviews have been summarized below for your convenience but t…

Harpera, a new skin Microbiopsy Punch for dermatological research
In April 2022, Trajan Scientific and Medical licensed a novel skin microbiopsy™ technology, dubbed Harpera™, developed by the University of Queensland (UQ). This technology, crafted by Prof. Tarl W. Prow, Prof. Peter So…
Skin Research Center Opens in UK
October 2023 — A news article on the digital magazine site Cosmetics & Toiletries announced that a new skin research center in the United Kingdom will advance skin care technologies and treatments. The new center ho…
join the Microbiopsy movement embraced by top organizations around the world.





want to evaluate Harpera?

explore our full range of microsampling products.
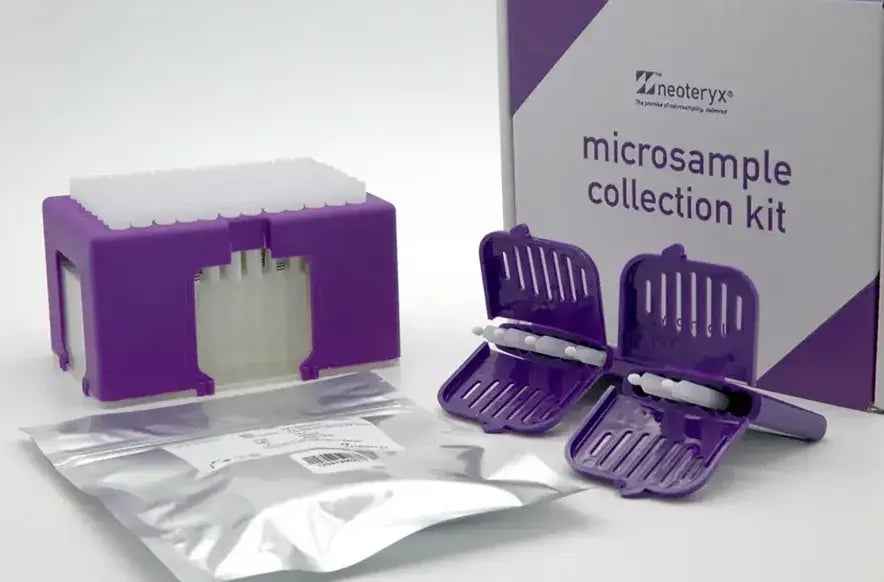
Mitra Microsampler
Visit Page
/hemaPEN%20images%20(approved)/IM_3238_RevB_RGB_72dpi_1024px-1.jpeg?width=1024&height=597&name=IM_3238_RevB_RGB_72dpi_1024px-1.jpeg)
hemaPEN DBS Next Generation
Visit Page

GenTegraRNA-NEO Solution
Visit Page
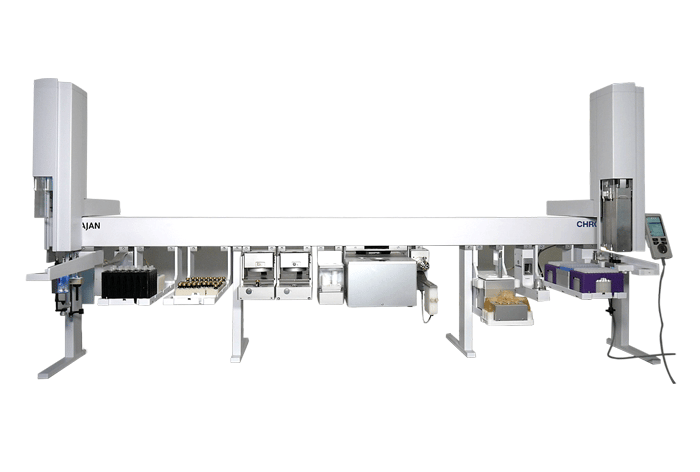
CHRONECT™ Accessioning and Processing
Visit Page
