Polaris Dawn Crew Uses Trajan's Microsampling Devices to Aid Research on Drug Effects in Space


Polaris Dawn Crew Uses Trajan's Microsampling Devices to Aid Research on Drug Effects in Space
September 11,2024
2
min read
Australia’s First Egg Count Kit: Reproductive Health Update


Australia’s First Egg Count Kit: Reproductive Health Update
August 30,2024
4
min read
Ghent University Isolates RNA in Dried Blood Microsamples


Ghent University Isolates RNA in Dried Blood Microsamples
May 31,2024
3
min read
CliniSciences Distributes Trajan Microsampling Devices in Europe


CliniSciences Distributes Trajan Microsampling Devices in Europe
January 9,2024
4
min read
Avenna Researches Patient-Centric Microsampling with Trajan Devices
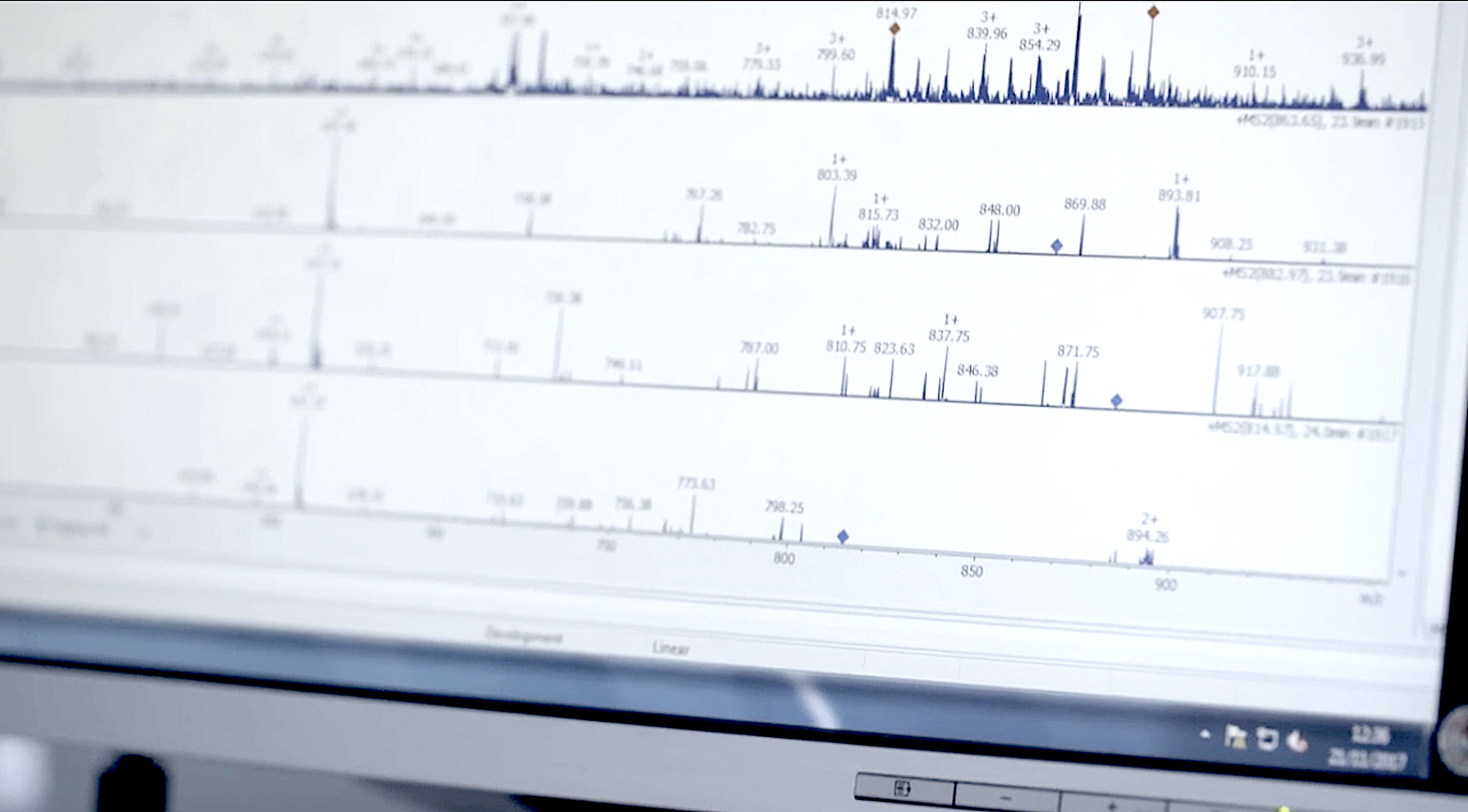

Avenna Researches Patient-Centric Microsampling with Trajan Devices
December 4,2023
4
min read
Trajan Distributes RNA Stabilization Solution for Mitra Microsampling


Trajan Distributes RNA Stabilization Solution for Mitra Microsampling
November 28,2023
3
min read
