Brazil Registers Neoteryx’s Mitra Devices for Specimen Collection


Brazil Registers Neoteryx’s Mitra Devices for Specimen Collection
November 16,2021
3
min read
Neoteryx's Mitra Devices for Specimen Collection Registered in S. Africa
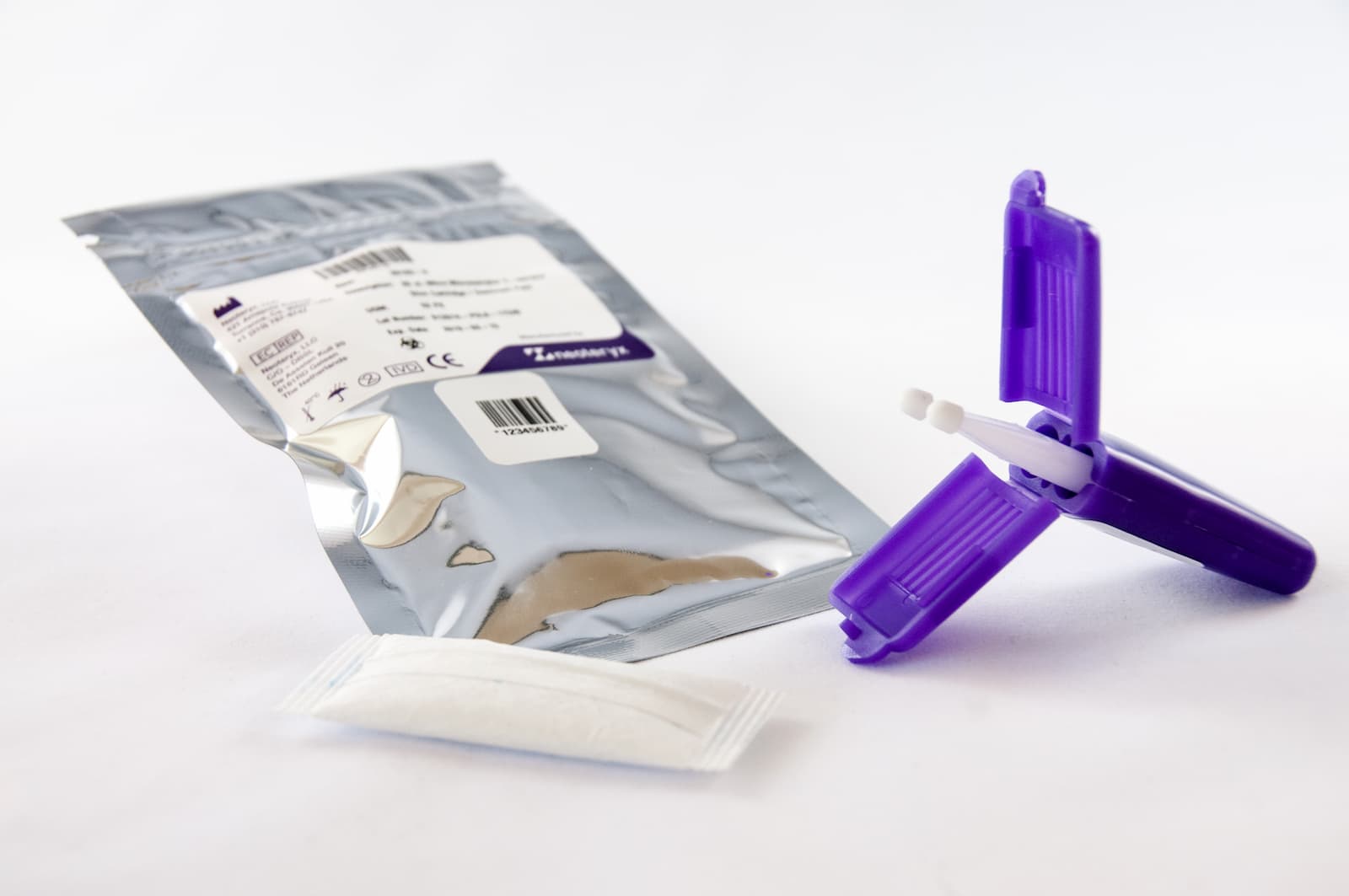

Neoteryx's Mitra Devices for Specimen Collection Registered in S. Africa
October 4,2021
4
min read
Telehealth, Telemedicine’s Rise Requires Remote Specimen Collection
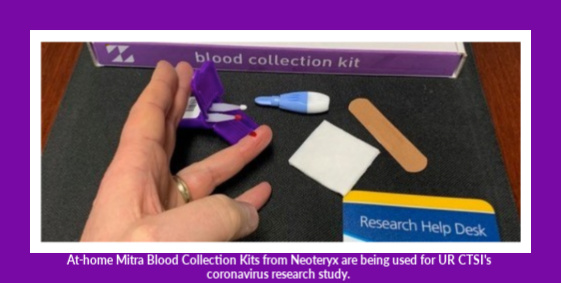

Telehealth, Telemedicine’s Rise Requires Remote Specimen Collection
December 14,2020
4
min read
