Share this
blood microsampling in pharma, clinical trials, and drug development: a smarter, simpler future
by Neoteryx Microsampling on Apr 18, 2019 3:23:00 AM
Scientists and clinicians working in pharmaceutical research or drug development and clinical trials face challenges in collecting blood samples. Blood sampling is a critical component in clinical research, from pre-clinical animal studies through all phases of clinical trials involving human study volunteers.
Yet, collecting blood samples using traditional venipuncture blood draws performed by a trained phlebotomist adds to the overall trial costs and is inconvenient for study volunteers.
Today there are smarter, simpler technologies available that enable remote blood microsampling in pharma drug development and clinical trials. Blood microsampling is part of a paradigm shift that is changing the way we design clinical trials and approach drug development research and healthcare. /full-tips-universal-anywhere-office.jpeg?width=822&height=479&name=full-tips-universal-anywhere-office.jpeg)
Welcome To The Blood Microsampling Revolution
The latest microsampling technologies include portable devices based on volumetric microsampling to facilitate remote, small-volume blood collection by anyone, anywhere.
These devices overcome some of the limitations of dried blood spot (DBS) cards or DBS filter papers, which have been reported to have issues with inconsistent sample volumes and hematocrit bias.
 Mitra® devices based on VAMS® technology can be used by study participants themselves to self-collect blood samples at home and away from the clinic.
Mitra® devices based on VAMS® technology can be used by study participants themselves to self-collect blood samples at home and away from the clinic.
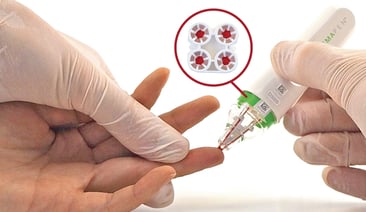 The hemaPEN® device is another volumetric microsampling tool that provides easy sampling beyond the confines of the clinic or research facility. This device can be used for assisted blood collection with a healthcare professional, though no special training is needed.
The hemaPEN® device is another volumetric microsampling tool that provides easy sampling beyond the confines of the clinic or research facility. This device can be used for assisted blood collection with a healthcare professional, though no special training is needed.
The hemaPEN simultaneously collects 4 DBS samples from a single subject or single source, with volumetric precision. This device overcomes the limitations of conventional DBS cards, consistently delivering four identical-volume samples of 2.74 µL each.
Running Person-Centric, Decentralized Clinical Trials
 To ensure the success of their clinical trials, the study designers, organizers and collaborators must increasingly prioritize the interests of their patients and participants. That means making the process of clinical trial participation as convenient, efficient and rewarding as possible.
To ensure the success of their clinical trials, the study designers, organizers and collaborators must increasingly prioritize the interests of their patients and participants. That means making the process of clinical trial participation as convenient, efficient and rewarding as possible.
Implementing microsampling in clinical trials is one simple way to do this. Offering an option for remote microsampling enables study volunteers and patients to participate from home, which is more comfortable and convenient. Trial staff can follow up with their participants remotely, using digital health communications such as email, video conferencing and secure website portals.
Allowing off-site, small-volume sampling makes things simpler for participants and trial managers alike, saving hassle in the short run and saving money in the long run.
When it comes to blood collection, new and improved microsampling methods are preferred by patients over outmoded methods, such as painful and stress-inducing venipuncture.
Recruitment, Retention, Adherence, and Compliance
Microsampling improves the process and experience of clinical trial participation. Making things easier and more comfortable for participants increases the likelihood of getting better results and streamlines the drug development process.
It increases the potential pool for recruitment, especially in geographical areas where travel or wet-blood shipment would have previously been cost-prohibitive.
It improves subject retention, as participants are more likely to stick with trials when it's more convenient and more pleasant to do so.
And it improves adherence and compliance with trial regimens, as both sampling and monitoring become much easier.
The Science Is Solid
 Microsampling methods have been shown to generate results that correlate with those generated by the gold standard associated with wet blood. As a quantitative method that collects precise volumes of dried blood, volumetric microsampling eliminates the hematocrit bias that plagued the imprecise samples associated with old-fashioned dried blood spot (DBS) cards and filter paper.
Microsampling methods have been shown to generate results that correlate with those generated by the gold standard associated with wet blood. As a quantitative method that collects precise volumes of dried blood, volumetric microsampling eliminates the hematocrit bias that plagued the imprecise samples associated with old-fashioned dried blood spot (DBS) cards and filter paper.
As seen in hundreds of peer-reviewed articles in the Microsampling Resource Library on the Neoteryx website, side-by-side studies have shown repeated success for blood microsampling, which is compatible with a wide range of analytes. Simply put, microsampling is the future of blood collection for clinical trials.
Smaller is Better, and It Is Working
The microsampling products team at Trajan Scientific and Medical has seen large and small customers in the fields of pharma, clinical trials, and drug development build tremendous momentum in their studies and programs using microsampling technology.
The big success stories show a typical and repeatable pattern of commitment, tenacity, rigor, and follow-through.
We invite you to explore the case studies and projects highlighted on our website and in our Resource Library to witness the success achieved by others. If you have questions, please reach out to our Microsampling Specialists - they'll show you how to replicate that success for yourself, in your own work.
Toward a Smarter, Simpler Future
Technology makes it possible to conduct clinical trials in remarkable new ways that defy old assumptions and expectations. Person-centric clinical trials, virtual clinical trials, and geography-agnostic drug development are all just beginning to make good on their tremendous promise. By "going remote" you can reach more diverse communities – and the people who may benefit most from the new drugs you are trialing.
When you arm yourself with new innovations such as blood microsampling solutions, you prepare yourself to succeed in a future of better pharma.
Contact a Microsampling Specialist today and take your first step toward success in a new landscape for clinical trials and drug development.
Share this
- Microsampling (206)
- Research, Remote Research (119)
- Venipuncture Alternative (105)
- Clinical Trials, Clinical Research (83)
- Mitra® Device (73)
- Therapeutic Drug Monitoring, TDM (51)
- Dried Blood Spot, DBS (39)
- Biomonitoring, Health, Wellness (30)
- Infectious Disease, Vaccines, COVID-19 (24)
- Blood Microsampling, Serology (23)
- Omics, Multi-Omics (21)
- Decentralized Clinical Trial (DCT) (20)
- Specimen Collection (18)
- Toxicology, Doping, Drug/Alcohol Monitoring, PEth (17)
- Skin Microsampling, Microbiopsy (14)
- hemaPEN® Device (13)
- Preclinical Research, Animal Studies (12)
- Pharmaceuticals, Drug Development (9)
- Harpera Device (7)
- Industry News, Microsampling News (5)
- Antibodies, MAbs (3)
- Company Press Release, Product Press Release (3)
- Environmental Toxins, Exposures (1)
- July 2025 (1)
- May 2025 (1)
- April 2025 (2)
- December 2024 (2)
- November 2024 (1)
- October 2024 (3)
- September 2024 (1)
- June 2024 (1)
- May 2024 (1)
- April 2024 (4)
- March 2024 (1)
- February 2024 (2)
- January 2024 (4)
- December 2023 (3)
- November 2023 (3)
- October 2023 (3)
- September 2023 (3)
- July 2023 (3)
- June 2023 (2)
- April 2023 (2)
- March 2023 (2)
- February 2023 (2)
- January 2023 (3)
- December 2022 (2)
- November 2022 (3)
- October 2022 (4)
- September 2022 (3)
- August 2022 (5)
- July 2022 (2)
- June 2022 (2)
- May 2022 (4)
- April 2022 (3)
- March 2022 (3)
- February 2022 (4)
- January 2022 (5)
- December 2021 (3)
- November 2021 (5)
- October 2021 (3)
- September 2021 (3)
- August 2021 (4)
- July 2021 (4)
- June 2021 (4)
- May 2021 (4)
- April 2021 (3)
- March 2021 (5)
- February 2021 (4)
- January 2021 (4)
- December 2020 (3)
- November 2020 (5)
- October 2020 (4)
- September 2020 (3)
- August 2020 (3)
- July 2020 (6)
- June 2020 (4)
- May 2020 (4)
- April 2020 (3)
- March 2020 (6)
- February 2020 (3)
- January 2020 (4)
- December 2019 (5)
- November 2019 (4)
- October 2019 (2)
- September 2019 (4)
- August 2019 (4)
- July 2019 (3)
- June 2019 (7)
- May 2019 (6)
- April 2019 (5)
- March 2019 (6)
- February 2019 (5)
- January 2019 (8)
- December 2018 (3)
- November 2018 (4)
- October 2018 (7)
- September 2018 (6)
- August 2018 (5)
- July 2018 (8)
- June 2018 (6)
- May 2018 (5)
- April 2018 (6)
- March 2018 (4)
- February 2018 (6)
- January 2018 (4)
- December 2017 (2)
- November 2017 (3)
- October 2017 (2)
- September 2017 (4)
- August 2017 (2)
- July 2017 (4)
- June 2017 (5)
- May 2017 (6)
- April 2017 (6)
- March 2017 (5)
- February 2017 (4)
- January 2017 (1)
- July 2016 (3)
- May 2016 (1)
- April 2016 (2)



Comments (1)