Share this
remote sampling and virtual clinical trials: a look back & into the future
by Neoteryx Microsampling on May 21, 2018 2:34:00 AM
The earliest clinical trial in history may have occurred in about 570 BC when King Nebuchadnezzar ruled Babylon. He tried to force Daniel and three companions to eat the royal diet of wine and meat. Daniel refused and the King let him eat nothing but vegetables and water. After a trial of 10 days, Daniel looked better nourished than his three friends who had consumed meat and wine. These "trial results" led the King to change the royal diet.
Most historians consider the first true clinical trial to be one that occurred in France in 1537. A military surgeon named Ambroise Paré conducted a trial of a novel therapy while treating wounded soldiers on the battlefield. When Dr. Paré ran out of boiling oil, the standard treatment for cauterizing wounds, he applied a mixture of turpentine, egg yolk, and oil of roses to seal wounds. The soldiers who received this trial therapy experienced less pain and healed faster than soldiers who had received the hot oil. This innovation led to a more effective wound treatment and fed the idea of advancement of health care through experimentation.
Another clinical trial milestone occurred in 1747, when ship doctor James Lind tried an experiment on sailors with scurvy. He divided them into groups and gave each group a different trial remedy. One group received vinegar or cider, while others received sulfuric acid mixed with alcohol or other potential therapies. One group received oranges and lemons.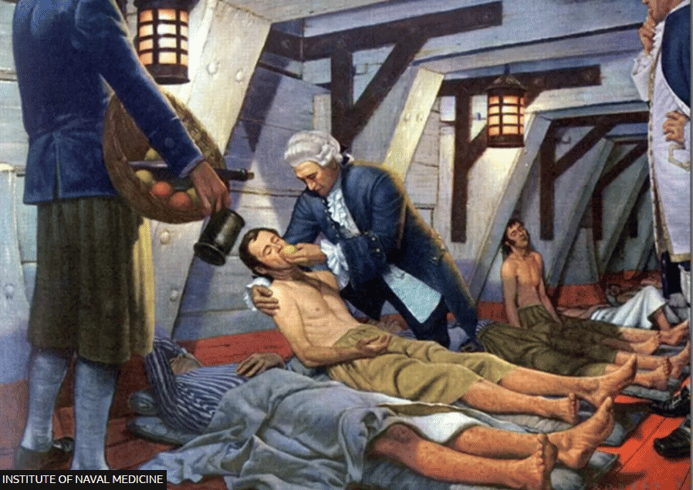
The sailors who received 2-3 citrus fruits a day recovered from scurvy and were ready to get back to work in a few days. The groups that received other experimental remedies didn't improve during the trial period. These results led Dr. Lind to give citrus fruits to the entire crew.
The success of this early "trial" eventually resulted in most ships carrying oranges, lemons or limes as a prevention and cure for scurvy during long voyages.
The Evolution of Clinical Trials: Advantages & Disadvantages
Today, clinical trials have evolved into complex randomized, double-blind studies that move through distinct phases. They are carefully regulated to assess efficacy of new drugs or devices and prevent harm. An advantage is that scientists are concerned about the safety of their study subjects, but a disadvantage is that inconvenience to trial volunteers can impact trial success.
Clinical trial participants typically have to travel miles on their own time to consult with trial staff at research centers or laboratories, where they undergo blood tests. They have to sit in waiting rooms, bored or nervous, while thinking about what they could be doing at work or at home.
Frequent and lengthy site visits for clinical trials can cause many people to drop out early. They may find the inconvenience is too disruptive to their daily lives. High dropout rates negatively impact trial success and can lead to higher clinical trial costs.
Other issues people face in clinical trials include uncomfortable procedures, such as venous blood draws, or a lack of clear communication from the trial managers about requirements. These issues can lead to problems with adherence or compliance with trial activities, and retention of trial subjects until the end of a trial.
A Trend Toward Decentralized or Virtual Clinical Trials
 One of the most difficult aspects of clinical studies has always been finding the requisite number of appropriate patients – and keeping them engaged in the trial.
One of the most difficult aspects of clinical studies has always been finding the requisite number of appropriate patients – and keeping them engaged in the trial.
Trial managers now ask, "How can we make our clinical trial more convenient and less burdensome for our study volunteers?" They are exploring ways to use digital technologies and remote devices to design virtual clinical trials that make it easier for volunteers to participate from home.
The virtual or decentralized approach can be a way to reach and recruit study volunteers from distant areas and diverse communities far away from city centers.
If study volunteers do not have to travel to trial sites in big city centers, they are more likely to participate – and stay committed to the trial until it ends. No matter where they are, virtual or digital technologies can be used to recruit and enroll them. The hurdle of geography is eliminated. And the time necessary to comply with the study is minimized compared to standard, onsite clinical trials.
Home Microsampling Kits Allow Trial Volunteers to Participate as "Armchair Scientists"
 With the availability of remote microsampling tools, like the Mitra® device people can collect their own samples at home with a quick finger-stick method using a lancet. In some cases, a health professional can conduct sample collection as part of a mobile unit.
With the availability of remote microsampling tools, like the Mitra® device people can collect their own samples at home with a quick finger-stick method using a lancet. In some cases, a health professional can conduct sample collection as part of a mobile unit.
Sample collection kits include instructions, devices, and all the supplies needed for easy blood collection from a fingertip. Study participants report that this approach is more convenient and involves less time, less pain, less stress...and less blood.
Microsampling devices deliver dried blood samples that can be sealed into a specimen bag and a mailing envelope that can be sent to the lab via standard mail. The laboratory processes and analyzes the dried microsampling following a DBS workflow to generate study data.
Clinical trial managers can follow up with their participants via smartphone apps, secure website portals, video conferencing and other telemedicine technologies.
Welcome to the modern era of clinical trials that not only advance medicine but make participation in important scientific studies easier as well.
Visit our resource page on Microsampling for Decentralized Clinical Trials to learn more:
Image credits: Institute of Naval Medicine, Shutterstock, Trajan, Neoteryx

Share this
- Microsampling (206)
- Research, Remote Research (119)
- Venipuncture Alternative (105)
- Clinical Trials, Clinical Research (83)
- Mitra® Device (73)
- Therapeutic Drug Monitoring, TDM (51)
- Dried Blood Spot, DBS (39)
- Biomonitoring, Health, Wellness (30)
- Infectious Disease, Vaccines, COVID-19 (24)
- Blood Microsampling, Serology (23)
- Omics, Multi-Omics (21)
- Decentralized Clinical Trial (DCT) (20)
- Specimen Collection (18)
- Toxicology, Doping, Drug/Alcohol Monitoring, PEth (17)
- Skin Microsampling, Microbiopsy (14)
- hemaPEN® Device (13)
- Preclinical Research, Animal Studies (12)
- Pharmaceuticals, Drug Development (9)
- Harpera Device (7)
- Industry News, Microsampling News (5)
- Antibodies, MAbs (3)
- Company Press Release, Product Press Release (3)
- Environmental Toxins, Exposures (1)
- July 2025 (1)
- May 2025 (1)
- April 2025 (2)
- December 2024 (2)
- November 2024 (1)
- October 2024 (3)
- September 2024 (1)
- June 2024 (1)
- May 2024 (1)
- April 2024 (4)
- March 2024 (1)
- February 2024 (2)
- January 2024 (4)
- December 2023 (3)
- November 2023 (3)
- October 2023 (3)
- September 2023 (3)
- July 2023 (3)
- June 2023 (2)
- April 2023 (2)
- March 2023 (2)
- February 2023 (2)
- January 2023 (3)
- December 2022 (2)
- November 2022 (3)
- October 2022 (4)
- September 2022 (3)
- August 2022 (5)
- July 2022 (2)
- June 2022 (2)
- May 2022 (4)
- April 2022 (3)
- March 2022 (3)
- February 2022 (4)
- January 2022 (5)
- December 2021 (3)
- November 2021 (5)
- October 2021 (3)
- September 2021 (3)
- August 2021 (4)
- July 2021 (4)
- June 2021 (4)
- May 2021 (4)
- April 2021 (3)
- March 2021 (5)
- February 2021 (4)
- January 2021 (4)
- December 2020 (3)
- November 2020 (5)
- October 2020 (4)
- September 2020 (3)
- August 2020 (3)
- July 2020 (6)
- June 2020 (4)
- May 2020 (4)
- April 2020 (3)
- March 2020 (6)
- February 2020 (3)
- January 2020 (4)
- December 2019 (5)
- November 2019 (4)
- October 2019 (2)
- September 2019 (4)
- August 2019 (4)
- July 2019 (3)
- June 2019 (7)
- May 2019 (6)
- April 2019 (5)
- March 2019 (6)
- February 2019 (5)
- January 2019 (8)
- December 2018 (3)
- November 2018 (4)
- October 2018 (7)
- September 2018 (6)
- August 2018 (5)
- July 2018 (8)
- June 2018 (6)
- May 2018 (5)
- April 2018 (6)
- March 2018 (4)
- February 2018 (6)
- January 2018 (4)
- December 2017 (2)
- November 2017 (3)
- October 2017 (2)
- September 2017 (4)
- August 2017 (2)
- July 2017 (4)
- June 2017 (5)
- May 2017 (6)
- April 2017 (6)
- March 2017 (5)
- February 2017 (4)
- January 2017 (1)
- July 2016 (3)
- May 2016 (1)
- April 2016 (2)


No Comments Yet
Let us know what you think