Share this
is dried blood a biohazard?
by Neoteryx Microsampling on Apr 9, 2018 6:06:00 AM
What Does "Biohazard" Legally Mean?
A biohazard is any biological material that may pose a risk to human health, including blood, whether wet or dry. Under OSHA’s Bloodborne Pathogens Standard and WHO/CDC guidelines, human blood and certain body fluids are classified as potentially infectious materials (PIMs), and must be handled, stored, transported, and disposed of with care.
How Infectious Is Dried Blood Compared to Wet Blood?
Wet blood presents a higher risk of transmitting pathogens because viruses and bacteria remain viable in liquid. Its why when shipping wet blood, regulatory issues arise along with expensive cold chain shipping costs.
This has contributed to dried blood sampling techniques as a preferable alternative. Dried blood samples in contrast, generally have a reduced viability of many pathogens but are not risk-free. Agents, such as Hepatitis B can survive in dried blood for days under certain conditions. OSHA/CDC guidelines remind us that even dried blood can transmit infection under unsafe handling, so handling it with non-gloved hands or open wounds is still a potential hazard.
How are dried blood samples shipped?
 Dried blood samples don't require cold storage/shipping or special handling. Allowing researchers and healthcare providers to send DBS collection kits to peoples home for remote sample collection.
Dried blood samples don't require cold storage/shipping or special handling. Allowing researchers and healthcare providers to send DBS collection kits to peoples home for remote sample collection.
With dried blood microsampling only a few drops of blood are required via fingerstick. The blood samples are collected and safely sealed inside a foil specimen bag.
The sealed specimen bag can be placed inside a shipping envelope and mailed to the laboratory via standard post.
Practical Precautions When Handling Dried Blood
- Always use gloves and, when needed, face protection to avoid accidental exposure.
- Disinfect surfaces before and after encounters with dried blood.
- Use biohazard-labeled, leak-proof containers for storage and transport.
- Follow OSHA or local regulatory guidelines for disposal.
- Maintain detailed chain of custody and labeling if samples are to be tested or transported.
Guidelines & Regulatory References
Working with wet blood, collected using traditional venipuncture methods, brings with it the full range of obligations associated with handling and disposing of biohazardous materials – not to mention the significant risks.
By contrast, dried blood handling is not considered to be as hazardous and, therefore, is not subjected to the same stringent biohazard requirements.
The Centers for Disease Control and Prevention (CDC) has updated its guidelines around handling and shipping dried blood specimens. While important safety precautions must be recognized and put in place, the guidelines for handling dried capillary blood specimens are not as rigorous as those for wet blood and other serious biohazards.
- OSHA’s Bloodborne Pathogens Standard (29 CFR 1910.1030)
- CDC guidelines for bloodborne infectious diseases
- WHO recommendations on safe handling of biological specimens
FAQs: Common Questions About Dried Blood and Biohazards
Dried blood may still contain bloodborne pathogens such as HIV, Hepatitis B, or Hepatitis C. However, once blood dries, the number of viable pathogens typically decreases over time. According to the CDC and OSHA, some viruses may survive for hours to days in dried blood, depending on environmental conditions like temperature and humidity. This means dried blood should always be handled with caution.
While the risk is lower than with fresh (wet) blood, dried blood can still carry infectious agents. The CDC notes that HIV does not survive long outside the body and is unlikely to be transmitted from dried blood. Hepatitis B and C viruses are more resilient and may survive longer, making proper precautions essential when handling dried blood.
Yes. Under OSHA and WHO guidelines, any material containing human blood—whether wet or dried—is considered a potential biohazard. This classification ensures consistent safety measures, such as gloves, proper labeling, and safe disposal practices.
Wet blood poses a higher infection risk because pathogens remain more viable in fluid. Dried blood, while less infectious, still warrants protective handling to avoid accidental exposure.
Summary
Though dried blood is not without risk, it’s generally less infectious than wet blood. But by incorporating current regulatory guidelines, taking practical precautions, and using informed judgment, dried blood handling can be made significantly safer for researchers, clinicians, and laboratory staff alike. Offering many around the globe the opportunity to avoid the costs and complexities of storing and shipping wet blood as a biohazard.
Per CDC guidelines, taking and sending capillary blood samples is easier, but the process may still have its challenges. Fortunately, Microsampling Collection Kits include detailed instructions and all the supplies needed for collecting volumetrically accurate blood samples.
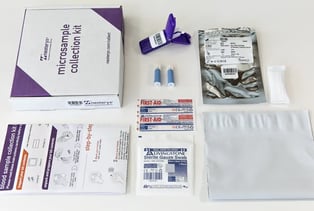 Virtually anyone can collect a remote blood sample using these finger-stick sampling kits, with minimal training.
Virtually anyone can collect a remote blood sample using these finger-stick sampling kits, with minimal training.
The kits can be fully customized to include instructions in multiple languages, with web links to training videos, or to meet other requirements of research study managers or clinical trial managers.

Image credits: Trajan, Neoteryx, iStock
Share this
- Microsampling (206)
- Research, Remote Research (119)
- Venipuncture Alternative (105)
- Clinical Trials, Clinical Research (83)
- Mitra® Device (73)
- Therapeutic Drug Monitoring, TDM (51)
- Dried Blood Spot, DBS (39)
- Biomonitoring, Health, Wellness (30)
- Infectious Disease, Vaccines, COVID-19 (24)
- Blood Microsampling, Serology (23)
- Omics, Multi-Omics (21)
- Decentralized Clinical Trial (DCT) (20)
- Specimen Collection (18)
- Toxicology, Doping, Drug/Alcohol Monitoring, PEth (17)
- Skin Microsampling, Microbiopsy (14)
- hemaPEN® Device (13)
- Preclinical Research, Animal Studies (12)
- Pharmaceuticals, Drug Development (9)
- Harpera Device (7)
- Industry News, Microsampling News (5)
- Antibodies, MAbs (3)
- Company Press Release, Product Press Release (3)
- Environmental Toxins, Exposures (1)
- July 2025 (1)
- May 2025 (1)
- April 2025 (2)
- December 2024 (2)
- November 2024 (1)
- October 2024 (3)
- September 2024 (1)
- June 2024 (1)
- May 2024 (1)
- April 2024 (4)
- March 2024 (1)
- February 2024 (2)
- January 2024 (4)
- December 2023 (3)
- November 2023 (3)
- October 2023 (3)
- September 2023 (3)
- July 2023 (3)
- June 2023 (2)
- April 2023 (2)
- March 2023 (2)
- February 2023 (2)
- January 2023 (3)
- December 2022 (2)
- November 2022 (3)
- October 2022 (4)
- September 2022 (3)
- August 2022 (5)
- July 2022 (2)
- June 2022 (2)
- May 2022 (4)
- April 2022 (3)
- March 2022 (3)
- February 2022 (4)
- January 2022 (5)
- December 2021 (3)
- November 2021 (5)
- October 2021 (3)
- September 2021 (3)
- August 2021 (4)
- July 2021 (4)
- June 2021 (4)
- May 2021 (4)
- April 2021 (3)
- March 2021 (5)
- February 2021 (4)
- January 2021 (4)
- December 2020 (3)
- November 2020 (5)
- October 2020 (4)
- September 2020 (3)
- August 2020 (3)
- July 2020 (6)
- June 2020 (4)
- May 2020 (4)
- April 2020 (3)
- March 2020 (6)
- February 2020 (3)
- January 2020 (4)
- December 2019 (5)
- November 2019 (4)
- October 2019 (2)
- September 2019 (4)
- August 2019 (4)
- July 2019 (3)
- June 2019 (7)
- May 2019 (6)
- April 2019 (5)
- March 2019 (6)
- February 2019 (5)
- January 2019 (8)
- December 2018 (3)
- November 2018 (4)
- October 2018 (7)
- September 2018 (6)
- August 2018 (5)
- July 2018 (8)
- June 2018 (6)
- May 2018 (5)
- April 2018 (6)
- March 2018 (4)
- February 2018 (6)
- January 2018 (4)
- December 2017 (2)
- November 2017 (3)
- October 2017 (2)
- September 2017 (4)
- August 2017 (2)
- July 2017 (4)
- June 2017 (5)
- May 2017 (6)
- April 2017 (6)
- March 2017 (5)
- February 2017 (4)
- January 2017 (1)
- July 2016 (3)
- May 2016 (1)
- April 2016 (2)

Comments (2)