the viability of Mitra devices for therapeutic drug monitoring
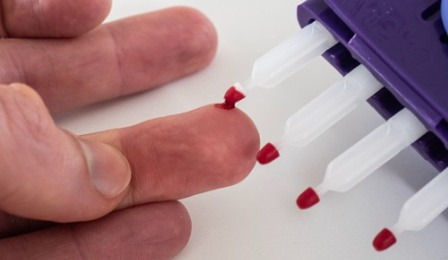

the viability of Mitra devices for therapeutic drug monitoring
Dec 16, 2024 1:32:11 PM
5
min read
the importance of clinical bridging for pharmacokinetic (PK) assays
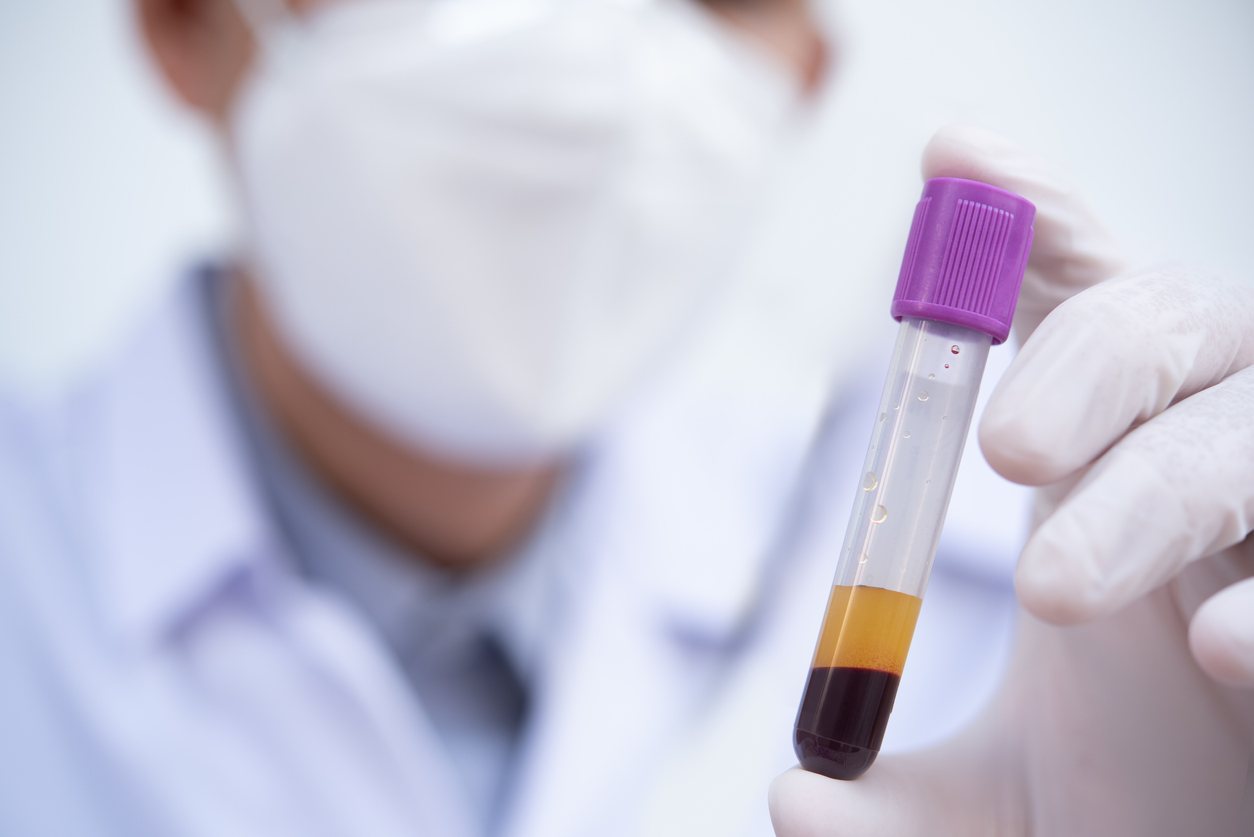

the importance of clinical bridging for pharmacokinetic (PK) assays
Mar 11, 2024 9:00:00 AM
6
min read
perceived patient adherence improves with app and remote microsampling


perceived patient adherence improves with app and remote microsampling
Nov 13, 2023 9:00:00 AM
6
min read
microsampling for patients on therapeutic drugs: Dr. Lemaitre


microsampling for patients on therapeutic drugs: Dr. Lemaitre
Oct 30, 2023 2:58:35 PM
6
min read
microsampling automation of hemaPEN for TDM of tacrolimus
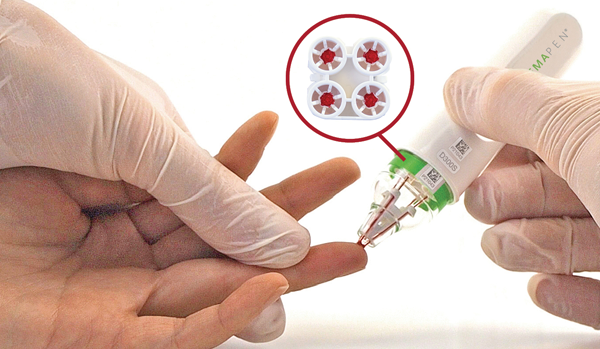

microsampling automation of hemaPEN for TDM of tacrolimus
Jun 12, 2023 9:00:00 AM
6
min read
monitoring blood lead levels using VAMS


monitoring blood lead levels using VAMS
Apr 17, 2023 9:00:00 AM
5
min read
