Share this
the importance of clinical bridging for pharmacokinetic (PK) assays
by James Rudge, PhD, Technical Director, Trajan on Mar 11, 2024 9:00:00 AM
An article by Hugues Chanteux et al at UCB Biopharma Belgium and two other institutions published in the November 2023 edition of The AAPS Journal described the use of Mitra® microsampling devices based on VAMS® technology in several clinical trials for development of an anticonvulsant called padsevonil (PSL). Their paper was entitled “Clinical Bridging Studies and Modeling Approach for Implementation of a Patient Centric Sampling Technique in Padsevonil Clinical Development.”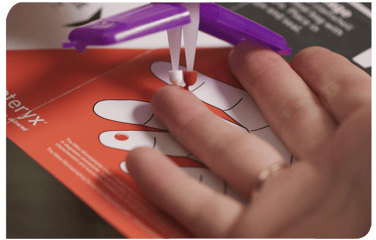 The research group conducted an extensive clinical bridging study involving over 2,500 paired blood samples from epilepsy patients and healthy volunteers.
The research group conducted an extensive clinical bridging study involving over 2,500 paired blood samples from epilepsy patients and healthy volunteers.
From their work they were able to conclude that the approach used for bridging was considered adequate by the Food and Drug Administration (FDA) to support Mitra devices as the sole method for PK sampling in future clinical trials.
Plasma Samples vs. Whole Blood Samples
Traditionally, the gold standard biological matrix for measuring the concentration of drugs in blood is plasma. Plasma is the straw-colored liquid which remains when the cellular intact portion of blood (hematocrit, or HCT) is removed either by centrifugation or filtration.
 There are good reasons for why drugs are traditionally analyzed from plasma rather than from whole blood.
There are good reasons for why drugs are traditionally analyzed from plasma rather than from whole blood.
One reason is that plasma is a less complex matrix, so there are less matrix interferences to mitigate when quantitating drug molecule concentrations. This is particularly important for LC-MS applications where matrix enhancement and suppression effects are commonly observed and need to be controlled.
Another reason is that protein free drugs in plasma are often what is available for peripheral cells to interact with, allowing pharmacological effects. As a result, it is useful to measure the change in free drug concentrations during pharmacokinetic studies.
Plasma is not without its drawbacks, however. First, a plasma assay does not take into account any partitioning that the drug has undertaken into the HCT. This is important with drugs that partition preferentially into the HCT where removal of the HCT will result in removal of a majority of the drug. There is a risk of underrepresenting the drug concentration in plasma-based drug studies.
Under these circumstances, measuring the drug concentration from blood which contains cells is vital to get a representative drug concentration. An example of such a class of drugs that associate primarily with the HCT are the calcineurin inhibiters, such as tacrolimus and cyclosporine.
For the vast majority of drugs, either the molecule partitions equally into the HCT, or partitioning favors the plasma fraction. This is why plasma has historically been the preferred matrix for measuring drug concentration levels for a range of drugs. Due to improvements in analytical technology, it is very possible to measure these drugs from whole blood, too.
Why Clinical Bridging?
However, if an assay has been initially validated in venous plasma, then clinical bridging needs to be conducted to understand what biases are seen when transitioning an assay to whole blood. Firstly, B:P partitioning can be dynamic. Under these circumstances, the partitioning can be concentration- and or time-dependent. Furthermore, drug concentrations may not be the same in the peripheral circulation compared to central circulation as sampled in venous blood.
 Therefore, it is important to check for any differences in drug concentration when it comes to the sampling site (e.g., fingertip vs. arm).
Therefore, it is important to check for any differences in drug concentration when it comes to the sampling site (e.g., fingertip vs. arm).
Finally, the impact of anticoagulants needs to be taken into consideration when uncoagulated capillary blood is collected and compared to venous plasma harvested from stabilized blood.
As discussed in the paper summarized here, the team at UCB Biopharma in Belgium used Mitra devices based on VAMS technology in several clinical trial phases during drug development of the anticonvulsant Padsevonil (PSL). The Mitra devices offered their study cohorts convenient remote sampling by enabling collection of < 30 µL blood samples at home from a finger-prick. The remote samples could be collected by study cohorts themselves or with assistance from a friend or family member.
The Mitra devices delivered dried blood samples for analysis, which was a benefit because dried blood is often considered safer and more stable than wet blood. For this reason, dried blood samples can typically be sent in the regular mail to laboratories for testing, which negates the need for cold chain shipping and storage. These benefits make remote, volumetric absorptive microsampling ideal for large multi-center clinical trials.
It was critical for the UCB Biopharma team to thoroughly understand the blood to plasma partitioning kinetics of the drug by conducting extensive clinical bridging. From this data they were able to develop an algorithm to compensate for the dynamic B:P partitioning that they observed.
Study Findings for Blood to Plasma Partitioning in PK Assays
Six clinical trials were conducted to understand the PK of PSL where analysis of the relationship between Mitra samples and venous plasma was investigated. Four of these trials were employed for method development and the final two (which included a phase 2 trial) for method validation.
- The subjects were from a mix of backgrounds and ethnicities and dependent on the clinical trial, the cohorts were either patients or heathy volunteers.
- In total, 3,048 paired samples were collected and analyzed.
- The extensive bridging work was conducted to support regulatory approval of a phase2b / phase 3 study where PK sampling was conducted solely with Mitra devices.
- Although the drug development program was halted for reasons unrelated to the bridging study, the group said, “the present bridging strategy was shared during a Type C interaction and was considered adequate by FDA to support use of Mitra as unique PK sampling approach in future clinical trials.”
- A time effect on B:P ratio was observed, where they saw an increase after dosing from 0.5-2 hour and this dropped down to its steady state value after around 4 hours.
- Other studies observed that the pre-dose B:P ratio was similar to the 4-hour steady state value.
- The group said that this could be explained by the different sampling sites that the blood was collected from.
- Blood from fingerpick sample collection is of a different consistency compared to blood obtained from venous collection. The UCB Biopharma group commented that blood collected from a finger-prick is a mixture of mainly arteriolar, venous and capillary blood rather than what we would classically consider as capillary blood. They explained that at the initial stages of measuring drug concentration over a PK, that arterial blood concentration can be higher than the venous concentration, due to efficient extraction of drugs from arterioles to tissue. Thus, during this distribution stage, it can lead to an apparent higher B:P. This phenomenon had also been described in other studies they cited.
- They developed a linear-mixed effect model based on clinical pharmacology studies. Using this and other data, they were able to adequately predict plasma from the dried blood VAMS samples with a mean bias of <9%.
- The in vivo B:P mirrored the in vitro data.
Study Authors’ Conclusions
The paper highlights the importance of clinical bridging to correlate between blood samples collected from a finger-prick and venous blood drawn from a vein in the arm.
- The approach was considered adequate by the FDA for PK sampling studies using just dried blood Mitra samples.
- The work sets the foundation for the implementation of the bridging for future studies.
Comments from Neoteryx Microsampling by Trajan
The work summarized here shows the successful implementation of using dried blood samples in a drug development program where over 2,500 paired samples were successfully collected. The clinical bridging data demonstrated that it was possible to use just dried blood samples for part of the study. The work also showed that both samples collected in clinic and remotely could be effectively correlated.
Additionally, this study highlighted the importance of the sampling site (i.e., fingertip vs. arm) and how the drug concentration can change between venous blood and capillary blood collected from a fingerpick, especially in the early stages of dosing. Understanding this phenomenon and mitigating it using statistical models from clinical bridging increases the likelihood of successful use of dried blood samples in future remote or hybrid clinical trials.
Readers are encouraged to refer to an excellent paper by Rowland and Emmons, which discusses in depth the impact of using dried blood in PK studies (a point that was also highlighted by the authors of this study.)
This article was summarized for our readers by James Rudge, PhD, Neoteryx Microsampling Technical Director, Trajan. This is curated content. To learn more about the important research outlined in this blog, visit the original article in The AAPS Journal.
Image Credits: Trajan, Neoteryx, iStock
Share this
- Microsampling (206)
- Research, Remote Research (119)
- Venipuncture Alternative (105)
- Clinical Trials, Clinical Research (83)
- Mitra® Device (73)
- Therapeutic Drug Monitoring, TDM (51)
- Dried Blood Spot, DBS (39)
- Biomonitoring, Health, Wellness (30)
- Infectious Disease, Vaccines, COVID-19 (24)
- Blood Microsampling, Serology (23)
- Omics, Multi-Omics (21)
- Decentralized Clinical Trial (DCT) (20)
- Specimen Collection (18)
- Toxicology, Doping, Drug/Alcohol Monitoring, PEth (17)
- Skin Microsampling, Microbiopsy (14)
- hemaPEN® Device (13)
- Preclinical Research, Animal Studies (12)
- Pharmaceuticals, Drug Development (9)
- Harpera Device (7)
- Industry News, Microsampling News (5)
- Antibodies, MAbs (3)
- Company Press Release, Product Press Release (3)
- Environmental Toxins, Exposures (1)
- July 2025 (1)
- May 2025 (1)
- April 2025 (2)
- December 2024 (2)
- November 2024 (1)
- October 2024 (3)
- September 2024 (1)
- June 2024 (1)
- May 2024 (1)
- April 2024 (4)
- March 2024 (1)
- February 2024 (2)
- January 2024 (4)
- December 2023 (3)
- November 2023 (3)
- October 2023 (3)
- September 2023 (3)
- July 2023 (3)
- June 2023 (2)
- April 2023 (2)
- March 2023 (2)
- February 2023 (2)
- January 2023 (3)
- December 2022 (2)
- November 2022 (3)
- October 2022 (4)
- September 2022 (3)
- August 2022 (5)
- July 2022 (2)
- June 2022 (2)
- May 2022 (4)
- April 2022 (3)
- March 2022 (3)
- February 2022 (4)
- January 2022 (5)
- December 2021 (3)
- November 2021 (5)
- October 2021 (3)
- September 2021 (3)
- August 2021 (4)
- July 2021 (4)
- June 2021 (4)
- May 2021 (4)
- April 2021 (3)
- March 2021 (5)
- February 2021 (4)
- January 2021 (4)
- December 2020 (3)
- November 2020 (5)
- October 2020 (4)
- September 2020 (3)
- August 2020 (3)
- July 2020 (6)
- June 2020 (4)
- May 2020 (4)
- April 2020 (3)
- March 2020 (6)
- February 2020 (3)
- January 2020 (4)
- December 2019 (5)
- November 2019 (4)
- October 2019 (2)
- September 2019 (4)
- August 2019 (4)
- July 2019 (3)
- June 2019 (7)
- May 2019 (6)
- April 2019 (5)
- March 2019 (6)
- February 2019 (5)
- January 2019 (8)
- December 2018 (3)
- November 2018 (4)
- October 2018 (7)
- September 2018 (6)
- August 2018 (5)
- July 2018 (8)
- June 2018 (6)
- May 2018 (5)
- April 2018 (6)
- March 2018 (4)
- February 2018 (6)
- January 2018 (4)
- December 2017 (2)
- November 2017 (3)
- October 2017 (2)
- September 2017 (4)
- August 2017 (2)
- July 2017 (4)
- June 2017 (5)
- May 2017 (6)
- April 2017 (6)
- March 2017 (5)
- February 2017 (4)
- January 2017 (1)
- July 2016 (3)
- May 2016 (1)
- April 2016 (2)



No Comments Yet
Let us know what you think