Share this
microsampling automation of hemaPEN for TDM of tacrolimus
by James Rudge, PhD, Technical Director, Trajan on Jun 12, 2023 9:00:00 AM
As more institutions onboard microsampling, the need to automate the handling and extraction of samples is increasing. In this blog we review an article by Caroline Monchaud et al at the CHU Limoges, France that was published in the May 2023 issue of the Journal of Chromatography B.
The paper discusses a successful analytical validation of hemaPEN® blood samples that was achieved for an automated extraction method for tacrolimus measurements using a Shimadzu CLAM-2030® and LCMS-8060®. The hemaPEN device is based on a volumetric microsampling technology that delivers 4 identical dried blood spot (DBS) samples./hemaPEN%20images%20(approved)/lightbox-hemapen-solo-3-xtr-brit-1-1.jpeg?width=761&height=333&name=lightbox-hemapen-solo-3-xtr-brit-1-1.jpeg)
Why automation?
Clinical chemistry laboratories are busy places. In many cases, they are processing thousands of blood tubes per day – each containing roughly 5-7 mL of wet blood – for the measurement of a huge variety of analytes, including cardiac and liver panels, electrolytes, drug levels, and more.
Much of the workflow within these laboratories is fully automated, employing barcode scanning that ensures sample identification, from initial sample processing through to the preparation of serum before being sent via conveyor belts (sometimes referred to as "streets") to analyzers. The analyzers utilize techniques such as spectrophotometry, immunoturbidimetry and chemiluminescence immunoassay (CLIA). The data from these instruments is then automatically reported into data systems for review.
A class of molecules which are measured using CLIA are calcineurin inhibitors. These therapeutic drugs are immunosuppressants, which are used in combination with other therapies for graft protection in transplant patients. Although CLIA is used in laboratories for measurement of calcineurin inhibitors, another popular (and sometimes preferred technique), is LC-MS/MS.
Automation Challenges for LC-MS/MS
Unlike many CLIA analyzers, LC-MS/MS instruments are not fully automated. This often leads to manual sample preparation of blood samples in many laboratories. This is not only time-consuming, but care must be taken to ensure human errors are eliminated in handling and processing the blood samples.
It is hoped that fully automating samples for LC-MS/MS will help minimize errors and free up laboratory technicians for other tasks. Indeed, there are several automation options available for processing blood samples for LC-MS/MS. These include parallel 96 well processing stations as well as serial preparation and direct inject configurations.
Another automation solution, a CLAM-2030 (innovated by Shimadzu), is a fully automated module for sample preparation for biofluids such as blood, urine, and plasma. Like a standard clinical chemistry analyzer, the CLAM-2030 can accept blood tubes and automatically prepare the samples for analysis by LC-MS/MS. At the time of writing, the CLAM-2030 is a research use only product.
Automation Challenges for Dried Blood Samples
Since the COVID-19 Pandemic, the demand for remote capillary microsamples has increased. There has been huge interest in remote microsampling in the field of organ transplantation, where therapeutic drug monitoring (TDM) is critical.
/hemaPEN%20in%20the%20Lab_FEB%202023%20(approved)/opening-hemapen.jpg?width=3960&height=2640&name=opening-hemapen.jpg) Currently, there are over 25 manuscripts published on Trajan’s hemaPEN® and the company's other microsampling products. However, within the vast majority of these papers, the sample extraction methodology described is manual in nature and not automated.
Currently, there are over 25 manuscripts published on Trajan’s hemaPEN® and the company's other microsampling products. However, within the vast majority of these papers, the sample extraction methodology described is manual in nature and not automated.
There is an excellent review of microsampling for TDM (including transplant drugs) by Phillip Morgan, who is based at Kings College Hospital London. In a section on the current challenges of automating dried microsamples, Dr. Morgan says, “Small sample substrates must be meticulously extracted from holders, with awkward manipulation in the laboratory; hence, compatibility with automated systems is advantageous.”
Automation of hemaPEN Samples for TDM of Tacrolimus
To address challenges observed when trying to automate the extraction and analysis of volumetrically collected blood samples, the team at CHU Limoges decided to work on automating dried blood microsamples from hemaPEN devices and interfacing these on a CLAM-2030 connected to a Shimadzu LC-MS/MS.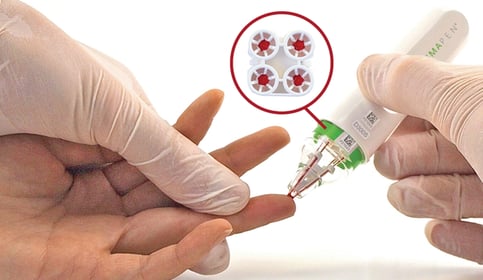
The hemaPEN is an easy-to-use dried blood microsampling device that collects 4 volumetrically identical capillary samples (2.74µL each) from a single finger-stick. A quick finger-stick method is much less stressful than traditional venous blood draws, making it ideal for therapeutic drug monitoring (TDM) programs.
Once collected, the samples are transferred to a cartridge of 4 dried blood spots (DBS) on pre-punched disks inside the hemaPEN.
Highlights from the Microsampling Automation Study
- Wet venous samples and dried capillary samples were prepared; the dried blood samples were either processed manually or were added to filtration vials and added to the CLAM-2030® for automated extraction. In both cases, the dried samples were extracted using MeOH/H2O 80:20 v/v containing the internal standard.
- Both the manual and automated dried blood extractions were compared to venous wet blood samples as well as being compared to each other.
- The dried extraction methods were validated in accordance with European Medicines Agency (EMA) guidelines on bioanalytical validation and in accordance with the International Association for Therapeutic Drug Monitoring and Clinical Toxicology (IATDMCT) guidelines.
- This automated extraction method met the calibration linearity, carry-over, selectivity, intraday and inter-day accuracy and precision acceptance criteria.
- No hematocrit (HCT) effect was observed (HCT range = 26-60%).
- The samples showed two-weeks’ stability at room temperature, and +60 °C over a period of 3 days. These stability results prompted the researchers to comment that the “use of hemaPEN for the therapeutic drug monitoring is compatible with real life conditions.”
- No interferences were observed when samples were spiked with about 40 potential co-medications, including oral contraceptives.
- This automated extraction method met the calibration linearity, carry-over, selectivity, intraday and inter-day accuracy and precision acceptance criteria.
- A clinical validation (called TAC-KIT_EASY) was then conducted on both renal and liver transplant patients (n=28). Capillary samples were collected with hemaPEN and analyzed using both manual and automated extraction methods. These were then compared to venipuncture samples that were run on the standard laboratory assay.
- Correlation was high between the wet and dried samples (r = 0.93 [manual] and 0.87 [automated]).
- Biases between the wet and dried samples were between -13.0% and 18.6% for manual extraction and -5% and 25.3% for automated extraction.
- Patient responses to the hemaPEN: The majority (90%) reported that the capillary sampling with hemaPEN was not painful. Almost all preferred the capillary sampling with a finger-stick technique versus the venous sampling from a blood draw in the arm.
- Not all samples met preanalytical requirements. It was thought that this was in part due to a lack of nurse practitioner experience in sample collection with the device. It was also thought that this may have been a contributing factor to the biases observed. However, the observed biases could also have been due to the fact that the DBS method used a different extraction technique compared to the whole blood assay.
- Correlation was high between the wet and dried samples (r = 0.93 [manual] and 0.87 [automated]).
Neoteryx Comments
/hemaPEN%20in%20the%20Lab_FEB%202023%20(approved)/hemapen-dbs-in-rack.jpg?width=486&height=324&name=hemapen-dbs-in-rack.jpg) Automating assays from microsamples is a challenge, yet it is important to validate assays for toxicology studies and therapeutic drug monitoring (TDM) programs.
Automating assays from microsamples is a challenge, yet it is important to validate assays for toxicology studies and therapeutic drug monitoring (TDM) programs.
The group from CHU Limoges showed a successful validation of the hemaPEN samples through both manual and automation sample extraction protocols for the analysis of tacrolimus.
Furthermore, an excellent correlation between wet and dried sample extracts was observed.
Although biases were seen between wet and dried samples, the differences in the wet extraction method and the dried method may well have contributed. Indeed, we observed something similar around 2015, in a research collaboration with two hospitals in the UK, when we were developing extraction methodology for Cyclosporin (unpublished data). Indeed, by changing the original organic extraction to a method based on the wet method (employing protein precipitation of an aqueous extraction), the resultant data was far more closely aligned.
This study was summarized for our readers by James Rudge, PhD, Technical Director. This is curated content. To learn more about the important research outlined in this blog, visit the original article in the Journal of Chromatography.
More information about microsampling for TDM can be found via our Microsampling for Drug Monitoring page.

Image Credits: Trajan, Neoteryx
Share this
- Microsampling (206)
- Research, Remote Research (119)
- Venipuncture Alternative (105)
- Clinical Trials, Clinical Research (83)
- Mitra® Device (73)
- Therapeutic Drug Monitoring, TDM (51)
- Dried Blood Spot, DBS (39)
- Biomonitoring, Health, Wellness (30)
- Infectious Disease, Vaccines, COVID-19 (24)
- Blood Microsampling, Serology (23)
- Omics, Multi-Omics (21)
- Decentralized Clinical Trial (DCT) (20)
- Specimen Collection (18)
- Toxicology, Doping, Drug/Alcohol Monitoring, PEth (17)
- Skin Microsampling, Microbiopsy (14)
- hemaPEN® Device (13)
- Preclinical Research, Animal Studies (12)
- Pharmaceuticals, Drug Development (9)
- Harpera Device (7)
- Industry News, Microsampling News (5)
- Antibodies, MAbs (3)
- Company Press Release, Product Press Release (3)
- Environmental Toxins, Exposures (1)
- July 2025 (1)
- May 2025 (1)
- April 2025 (2)
- December 2024 (2)
- November 2024 (1)
- October 2024 (3)
- September 2024 (1)
- June 2024 (1)
- May 2024 (1)
- April 2024 (4)
- March 2024 (1)
- February 2024 (2)
- January 2024 (4)
- December 2023 (3)
- November 2023 (3)
- October 2023 (3)
- September 2023 (3)
- July 2023 (3)
- June 2023 (2)
- April 2023 (2)
- March 2023 (2)
- February 2023 (2)
- January 2023 (3)
- December 2022 (2)
- November 2022 (3)
- October 2022 (4)
- September 2022 (3)
- August 2022 (5)
- July 2022 (2)
- June 2022 (2)
- May 2022 (4)
- April 2022 (3)
- March 2022 (3)
- February 2022 (4)
- January 2022 (5)
- December 2021 (3)
- November 2021 (5)
- October 2021 (3)
- September 2021 (3)
- August 2021 (4)
- July 2021 (4)
- June 2021 (4)
- May 2021 (4)
- April 2021 (3)
- March 2021 (5)
- February 2021 (4)
- January 2021 (4)
- December 2020 (3)
- November 2020 (5)
- October 2020 (4)
- September 2020 (3)
- August 2020 (3)
- July 2020 (6)
- June 2020 (4)
- May 2020 (4)
- April 2020 (3)
- March 2020 (6)
- February 2020 (3)
- January 2020 (4)
- December 2019 (5)
- November 2019 (4)
- October 2019 (2)
- September 2019 (4)
- August 2019 (4)
- July 2019 (3)
- June 2019 (7)
- May 2019 (6)
- April 2019 (5)
- March 2019 (6)
- February 2019 (5)
- January 2019 (8)
- December 2018 (3)
- November 2018 (4)
- October 2018 (7)
- September 2018 (6)
- August 2018 (5)
- July 2018 (8)
- June 2018 (6)
- May 2018 (5)
- April 2018 (6)
- March 2018 (4)
- February 2018 (6)
- January 2018 (4)
- December 2017 (2)
- November 2017 (3)
- October 2017 (2)
- September 2017 (4)
- August 2017 (2)
- July 2017 (4)
- June 2017 (5)
- May 2017 (6)
- April 2017 (6)
- March 2017 (5)
- February 2017 (4)
- January 2017 (1)
- July 2016 (3)
- May 2016 (1)
- April 2016 (2)


Comments (1)