microbiopsy punch may aid molecular profiling in dermatology
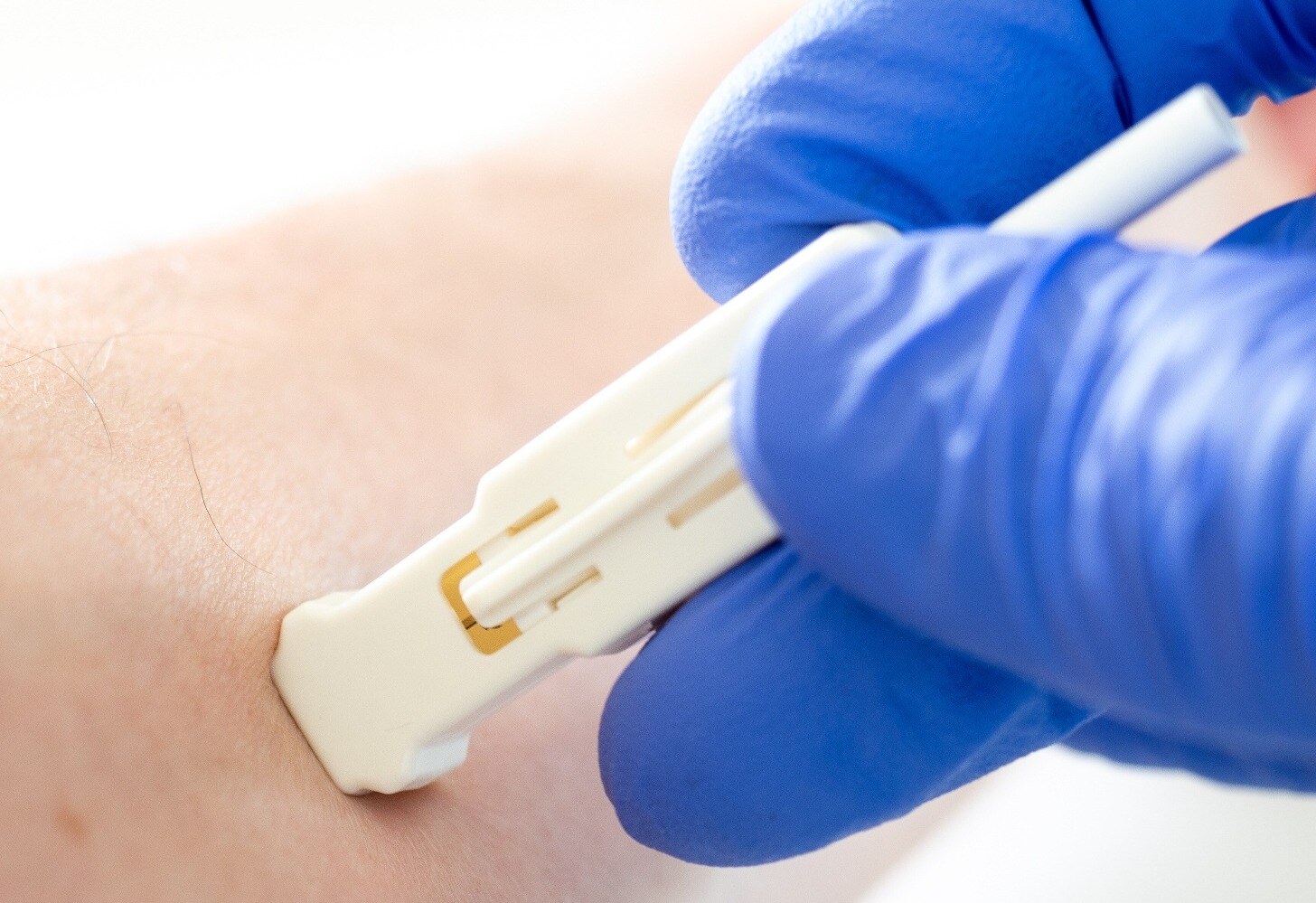

microbiopsy punch may aid molecular profiling in dermatology
Sep 20, 2024 10:29:00 AM
2
min read
expert Q&A: Harpera Microbiopsy Punch enables study of atopic dermatitis


expert Q&A: Harpera Microbiopsy Punch enables study of atopic dermatitis
May 13, 2024 10:06:27 AM
5
min read
Promega Publishes App Note on RNA Studies Using Mitra Devices


Promega Publishes App Note on RNA Studies Using Mitra Devices
Apr 18, 2024 3:44:28 PM
2
min read
saliva microsampling to monitor oral fluid for epilepsy


saliva microsampling to monitor oral fluid for epilepsy
Feb 12, 2024 9:00:00 AM
5
min read
Podcast: microsampling in the bioanalytical laboratory


Podcast: microsampling in the bioanalytical laboratory
Feb 5, 2024 9:00:00 AM
11
min read
Podcast: microsampling in proteomics & multi-center studies


Podcast: microsampling in proteomics & multi-center studies
Jan 29, 2024 9:00:00 AM
12
min read
