Share this
saliva microsampling to monitor oral fluid for epilepsy
by James Rudge, PhD, Technical Director, Trajan on Feb 12, 2024 9:00:00 AM
An article by Valentina Franco et al at several institutions in Italy and Norway published in the July 2023 edition of Pharmaceutics described a validated method for measuring an antiepileptic medicine from oral fluid in saliva samples collected using Mitra® microsampling devices based on VAMS® technology. Their paper was entitled “Therapeutic Salivary Monitoring of Perampanel in Patients with Epilepsy Using a Volumetric Absorptive Microsampling Technique.”
 Their study demonstrated that perampanel could be reliably monitored from dried saliva samples self-collected by patients at home. The study authors concluded that the method showed adequate bioanalytical performances and "holds great potential as an alternative strategy to support domiciliary TDM in patients with epilepsy treated with perampanel according to the simplicity of sample collection."
Their study demonstrated that perampanel could be reliably monitored from dried saliva samples self-collected by patients at home. The study authors concluded that the method showed adequate bioanalytical performances and "holds great potential as an alternative strategy to support domiciliary TDM in patients with epilepsy treated with perampanel according to the simplicity of sample collection."
Epilepsy and Perampanel
According to the World Health Organization (WHO), around 50 million people globally are living with epilepsy, with 80% living in low- and middle-income countries. The causes of this neurological disorder can include issues during childbirth, head trauma, infection of the brain, genetic abnormalities, stroke, and brain tumors. It is thought that 25% of epilepsy cases could be averted by taking measures to prevent some of the known causes, such as head injuries, adequate perinatal care, fever-reducing drugs, reducing risk factors around stroke and elimination of parasites in tropical areas, which can lead to brain infections, such as neurocysticercosis.
It is thought that nearly 70% of people living with epilepsy could be seizure free if they had access to appropriate antiseizure medicines. There are roughly 30 antiepileptic drugs (AEDs) currently on the market, one of which is perampanel (PER), an oral suspension medication that is sold under different brand names. The action or mechanism of PER is not fully understood; however, it is known that it acts as a selective antagonist of the postsynaptic ionotropic alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) glutamate receptor. It is also known that the glutamate is a major excitatory neurotransmitter. The current working hypothesis is that the AMPA receptor antagonism can reduce over stimulation of AMPA, thus inhibiting seizures.
In both Europe and the United States, PER is approved as an antiepileptic for adults and children over 12, though the types of seizures the drug is approved for differs with the age of the individual. Clinical trials using healthy volunteers showed that the elimination half-life was 53-136 hours, however, the clearance rate is affected by certain co-medications. For example, if PER is co-administered with another epilepsy drug, carbamazepine, then the total clearance rate of PER increases by three times. Similar effects are also seen when co-medicated with phenytoin or oxcarbazepine. As a result, it is important to therapeutically monitor PER in individuals, especially those on certain comedications.
The paper by Valentina Franco et al references two previously published studies where it is stated that therapeutic drug monitoring (TDM) of PER could be beneficial.
Salivary Microsampling for TDM
For most therapeutic drug monitoring, plasma is the standard matrix used and venipuncture is the standard means of collecting the required blood samples. Oral fluid (saliva), on the other hand, presents an intriguing potential alternative matrix for TDM. The major advantage of saliva is that the collection method is non-invasive, which is beneficial for needle-phobic and vulnerable cohorts, such as young children. Moreover, oral fluid can be easily collected by non-trained personnel.
However, use of saliva for therapeutic monitoring is not without its challenges. One challenge is that analytes are sometimes at a lower concentration in this matrix, making detection and quantitation challenging. Another challenge involves correlating serum or plasma equivalent values to those found in saliva, though the authors did reference another study where saliva had been successfully correlated with plasma for antiseizure medications. As with all wet matrices, there can also be concerns pertaining to compound stability.
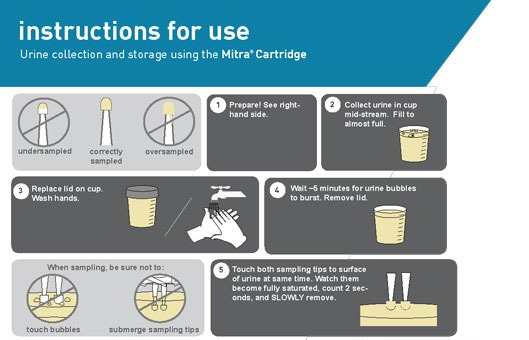 To address some of these challenges, Franco et al considered the use of volumetric absorptive microsampling with remote Mitra microsampling devices.
To address some of these challenges, Franco et al considered the use of volumetric absorptive microsampling with remote Mitra microsampling devices.
The advantages of this remote microsampling technology are that it enables home sampling, and it is designed to collect and store dried samples that can be sent via standard mail at room temperature with good stability.
The study authors believed this approach was well-suited for convenient, remote, and reliable sampling for TDM. They were further encouraged by case studies in the literature that showed dried blood collection using volumetric absorptive microsampling had been successfully used for antiepileptic research. They also learned that the VAMS technique had been successfully used for alternative fluid collection, including urine and saliva.
With this in mind, Franco et al embarked on their study to validate a dried salivary VAMS assay for TDM of PER.
Achievements of the Salivary PER Study
The researchers conducted a successful validation of salivary monitoring of PER from saliva microsamples using VAMS technology and LC-MS/MS in accordance with European Medicines Agency Guideline on Bioanalytical Method Validation.
Study Highlights Include:
- Inter- and intra-day precision was 12.1% and accuracy was within 85.6-103.2%
- Mean extraction recoveries of the LOQ, low, medium, and high QC values was: 97.2%, 81.3%, 98.7 and 96.5%
- Proven stability was observed for samples stored at room temperature for 72 hr, -20 °C for 1 month as well as 3 freeze-thaw cycles.
- No interference peaks were observed from 6 healthy volunteers or from patients taking the following concomitant anti-seizure medications: cannabidiol, carbamazepine, ethosuximide, lamotrigine, phenobarbital, topiramate, and vigabatrin.
- Solid and satisfactory accuracy and precision was seen for epilepsy patient samples collected on Mitra-VAMS devices and was found to be reliable for the quantification of PER in oral fluid.
- Saliva was found be easily collected on the Mitra-VAMS devices.
- The stability data highlighted the benefits of this approach as an alternative strategy to support patients with epilepsy by simplifying sample collection.
- Oral fluid sampling using VAMS technology could be effective for other antiepileptic drugs too; however, more studies would need to be conducted to validate the approach on these medications.
Final Thoughts on Saliva Microsampling
As LC-MS/MS instrumentation continues to improve in terms of sensitivity and specificity, coupling it with microsamples taken from alternative fluids provides an intriguing new avenue for drug and biomarker analysis. The non-invasive nature of saliva sample collection is an attractive option and an important biofluid to consider for future analysis. Moreover, if more studies show corroborative evidence of good correlation between serum and saliva levels, then saliva may play an even greater role in TDM in the future.
Furthermore, coupling LC-MS/MS studies to self-sampling using dried matrix collection devices vastly increases the options patients and study volunteers have for providing high-quality samples to the lab for analysis. This study demonstrates how effective oral fluid (saliva) samples collected on Mitra-VAMS devices can be for such applications.
This blog includes content curated from a published study paper that was summarized for our readers by James Rudge, PhD, Microsampling Technical Director, Trajan. To learn more about the important research discussed here, please visit the original article in Pharmaceutics.
Explore our resources on microsampling for therapeutic drug monitoring: 

Image Credits: Trajan, iStock
Share this
- Microsampling (206)
- Research, Remote Research (119)
- Venipuncture Alternative (105)
- Clinical Trials, Clinical Research (83)
- Mitra® Device (73)
- Therapeutic Drug Monitoring, TDM (51)
- Dried Blood Spot, DBS (39)
- Biomonitoring, Health, Wellness (30)
- Infectious Disease, Vaccines, COVID-19 (24)
- Blood Microsampling, Serology (23)
- Omics, Multi-Omics (21)
- Decentralized Clinical Trial (DCT) (20)
- Specimen Collection (18)
- Toxicology, Doping, Drug/Alcohol Monitoring, PEth (17)
- Skin Microsampling, Microbiopsy (14)
- hemaPEN® Device (13)
- Preclinical Research, Animal Studies (12)
- Pharmaceuticals, Drug Development (9)
- Harpera Device (7)
- Industry News, Microsampling News (5)
- Antibodies, MAbs (3)
- Company Press Release, Product Press Release (3)
- Environmental Toxins, Exposures (1)
- July 2025 (1)
- May 2025 (1)
- April 2025 (2)
- December 2024 (2)
- November 2024 (1)
- October 2024 (3)
- September 2024 (1)
- June 2024 (1)
- May 2024 (1)
- April 2024 (4)
- March 2024 (1)
- February 2024 (2)
- January 2024 (4)
- December 2023 (3)
- November 2023 (3)
- October 2023 (3)
- September 2023 (3)
- July 2023 (3)
- June 2023 (2)
- April 2023 (2)
- March 2023 (2)
- February 2023 (2)
- January 2023 (3)
- December 2022 (2)
- November 2022 (3)
- October 2022 (4)
- September 2022 (3)
- August 2022 (5)
- July 2022 (2)
- June 2022 (2)
- May 2022 (4)
- April 2022 (3)
- March 2022 (3)
- February 2022 (4)
- January 2022 (5)
- December 2021 (3)
- November 2021 (5)
- October 2021 (3)
- September 2021 (3)
- August 2021 (4)
- July 2021 (4)
- June 2021 (4)
- May 2021 (4)
- April 2021 (3)
- March 2021 (5)
- February 2021 (4)
- January 2021 (4)
- December 2020 (3)
- November 2020 (5)
- October 2020 (4)
- September 2020 (3)
- August 2020 (3)
- July 2020 (6)
- June 2020 (4)
- May 2020 (4)
- April 2020 (3)
- March 2020 (6)
- February 2020 (3)
- January 2020 (4)
- December 2019 (5)
- November 2019 (4)
- October 2019 (2)
- September 2019 (4)
- August 2019 (4)
- July 2019 (3)
- June 2019 (7)
- May 2019 (6)
- April 2019 (5)
- March 2019 (6)
- February 2019 (5)
- January 2019 (8)
- December 2018 (3)
- November 2018 (4)
- October 2018 (7)
- September 2018 (6)
- August 2018 (5)
- July 2018 (8)
- June 2018 (6)
- May 2018 (5)
- April 2018 (6)
- March 2018 (4)
- February 2018 (6)
- January 2018 (4)
- December 2017 (2)
- November 2017 (3)
- October 2017 (2)
- September 2017 (4)
- August 2017 (2)
- July 2017 (4)
- June 2017 (5)
- May 2017 (6)
- April 2017 (6)
- March 2017 (5)
- February 2017 (4)
- January 2017 (1)
- July 2016 (3)
- May 2016 (1)
- April 2016 (2)

Comments (1)