Share this
coronavirus tests and studies using at-home collection kits
by Neoteryx Microsampling on Apr 29, 2020 9:37:57 AM
The increasing number of people testing positive for COVID-19 — the infection caused by the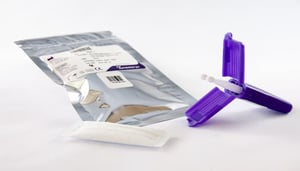 novel coronavirus called SARS-CoV-2 — demands easy-to-use remote sampling kits. Several organizations have been distributing different types of kits to facilitate both rapid testing of active COVID-19 infections and longer-term research studies of antibodies in blood that would help scientists understand immunity to the virus.
novel coronavirus called SARS-CoV-2 — demands easy-to-use remote sampling kits. Several organizations have been distributing different types of kits to facilitate both rapid testing of active COVID-19 infections and longer-term research studies of antibodies in blood that would help scientists understand immunity to the virus.
Kits that can be sent to remote study subjects from research organizations enable everyone to follow the much-needed social distancing rules recommended by the CDC and other organizations to avoid exposure to contagions.
Different Approaches Pursued as the COVID-19 Crisis Evolved
At the onset of the coronavirus outbreak, the focus was on using saliva swabs to test people who were experiencing symptoms to find out if they were actively infected with COVID-19. Many countries restricted this type of lab testing to specialized World Health Organization (WHO) facilities.
In the United States, some organizations set up more facilities for rapid testing using saliva swabs, but they experienced backlogs because so many people needed testing. Delays made it difficult for labs to provide results within 24-48 hours. This negated efforts to curb community transmission of the disease.
More recently, there has been an intensified effort to conduct more COVID-19 molecular studies using blood samples and other specimens, but high demand has caused global shortages in reagents, leading to more delays. The WHO also cites significant limitations to testing in low- and middle-income areas.
WHO’s lab testing strategy recommendations for coronavirus require countries without adequate testing capacity to send specimens of suspected coronavirus-positive subjects to WHO labs. Several companies developed home-based test kits to enable patients to collect their own samples at home and return their specimens to designated labs for testing.
Different Approaches to Specimen Collection for COVID-19 Studies
Researchers at the National Institutes of Health (NIH) and other organizations leveraged remote blood collection tools like the Mitra® microsampling device. The Mitra device is considered the gold standard for volumetric absorptive microsampling, which enables people to collect a precise-volume blood sample to be used by scientists in serological tests performed on the blood serum.
For serology studies, researchers analyze the blood samples for antibodies to the novel coronavirus. The presence of antibodies in a blood sample indicates that a person was previously infected with SARS-CoV-2 and recovered. In many cases, a person may not have displayed any symptoms, and may not have realized they had the infection. This information is critical to understanding the spread of the disease and how many undetected cases have occurred. Blood sample studies also provide information about immunity. These blood tests are unlike cotton swab saliva tests, which only identify if a person is actively infected with the virus at the time the swab is taken.
Companies Providing Devices and Technology for COVID-19 Testing
Other companies joined the battle to fight COVID-19 by leveraging remote specimen sampling technologies.
Some companies developed new home specimen collection kits per newer FDA guidelines that were updated to address the coronavirus pandemic. One company says it uses specific criteria before sending out its Coronavirus Assessment Tool Kit. The end-user must answer a series of questions while consulting with a provider. If they need COVID-19 swab testing and are eligible for home-based collection, their healthcare professional orders the kit, which is mailed to the end-user's home.
With this COVID test, a sample of saliva is taken from inside the cheek or roof of the mouth instead of the back of the throat or the nose. The patient ships the sample back to the company's lab for analysis.
Benefits of At-Home Specimen Collection & Remote Testing
With home-based specimen collection as an option, people stay at home during lockdowns for self-managed sample collection, which reduces their risk of cross-contamination. In some cases, blood sampling kits are mailed to study participants by research organizations that are conducting long-term serology studies. In other cases, saliva sampling kits are mailed to patients from a lab and the patients ship their saliva specimens back to the lab for testing.
An advantage of saliva testing that uses a cheek swab is that they aren’t prone to supply chain constraints as are nasal and throat swabs. This allows companies to reach more people.
Efficacy of At-Home Specimen Collection for COVID-19 Studies
Different types of scientific tests and studies are conducted for COVID-19. Two types are NAAT and serological studies. NAAT (nucleic acid amplification test) detects the presence of the SARS-CoV-2 virus that causes COVID-19. Evidence shows it has a high accuracy rate.
PCR (polymerase chain reaction) helps isolate infected patients and prevent transmission, so early testing is critical. The specimen testing process takes 3-4 hours, and results are available within several days. Evidence shows PCR testing alone has a 66.7% chance of detecting coronavirus within the first week. This discovery affirms the efficacy of recently developed home-based testing kits. RT-PCT tests from a few companies indicate comparable performance between throat swab tests and home saliva collection.
Serology (blood serum) studies detect antibodies in the body. The body produces IgM and IgG antibodies to fight foreign bodies called “antigens.” Once infected with COVID-19, the body produces antibodies to fight the infection. Serology studies help identify people who have developed antibodies to the SARS-CoV-2 virus, allowing them to serve as donors to infected patients. Serology studies can also help researchers develop scientific models of the virus for further investigations, including drug studies and vaccine development.
For more information on microsampling, click below:

Image Credit: Trajan
Share this
- Microsampling (206)
- Research, Remote Research (119)
- Venipuncture Alternative (105)
- Clinical Trials, Clinical Research (83)
- Mitra® Device (73)
- Therapeutic Drug Monitoring, TDM (51)
- Dried Blood Spot, DBS (39)
- Biomonitoring, Health, Wellness (30)
- Infectious Disease, Vaccines, COVID-19 (24)
- Blood Microsampling, Serology (23)
- Omics, Multi-Omics (21)
- Decentralized Clinical Trial (DCT) (20)
- Specimen Collection (18)
- Toxicology, Doping, Drug/Alcohol Monitoring, PEth (17)
- Skin Microsampling, Microbiopsy (14)
- hemaPEN® Device (13)
- Preclinical Research, Animal Studies (12)
- Pharmaceuticals, Drug Development (9)
- Harpera Device (7)
- Industry News, Microsampling News (5)
- Antibodies, MAbs (3)
- Company Press Release, Product Press Release (3)
- Environmental Toxins, Exposures (1)
- July 2025 (1)
- May 2025 (1)
- April 2025 (2)
- December 2024 (2)
- November 2024 (1)
- October 2024 (3)
- September 2024 (1)
- June 2024 (1)
- May 2024 (1)
- April 2024 (4)
- March 2024 (1)
- February 2024 (2)
- January 2024 (4)
- December 2023 (3)
- November 2023 (3)
- October 2023 (3)
- September 2023 (3)
- July 2023 (3)
- June 2023 (2)
- April 2023 (2)
- March 2023 (2)
- February 2023 (2)
- January 2023 (3)
- December 2022 (2)
- November 2022 (3)
- October 2022 (4)
- September 2022 (3)
- August 2022 (5)
- July 2022 (2)
- June 2022 (2)
- May 2022 (4)
- April 2022 (3)
- March 2022 (3)
- February 2022 (4)
- January 2022 (5)
- December 2021 (3)
- November 2021 (5)
- October 2021 (3)
- September 2021 (3)
- August 2021 (4)
- July 2021 (4)
- June 2021 (4)
- May 2021 (4)
- April 2021 (3)
- March 2021 (5)
- February 2021 (4)
- January 2021 (4)
- December 2020 (3)
- November 2020 (5)
- October 2020 (4)
- September 2020 (3)
- August 2020 (3)
- July 2020 (6)
- June 2020 (4)
- May 2020 (4)
- April 2020 (3)
- March 2020 (6)
- February 2020 (3)
- January 2020 (4)
- December 2019 (5)
- November 2019 (4)
- October 2019 (2)
- September 2019 (4)
- August 2019 (4)
- July 2019 (3)
- June 2019 (7)
- May 2019 (6)
- April 2019 (5)
- March 2019 (6)
- February 2019 (5)
- January 2019 (8)
- December 2018 (3)
- November 2018 (4)
- October 2018 (7)
- September 2018 (6)
- August 2018 (5)
- July 2018 (8)
- June 2018 (6)
- May 2018 (5)
- April 2018 (6)
- March 2018 (4)
- February 2018 (6)
- January 2018 (4)
- December 2017 (2)
- November 2017 (3)
- October 2017 (2)
- September 2017 (4)
- August 2017 (2)
- July 2017 (4)
- June 2017 (5)
- May 2017 (6)
- April 2017 (6)
- March 2017 (5)
- February 2017 (4)
- January 2017 (1)
- July 2016 (3)
- May 2016 (1)
- April 2016 (2)


No Comments Yet
Let us know what you think