The gold-standard in remote microsampling
Mitra® devices with VAMS® technology simplify remote specimen collection and microsampling, with formats that fit every budget.
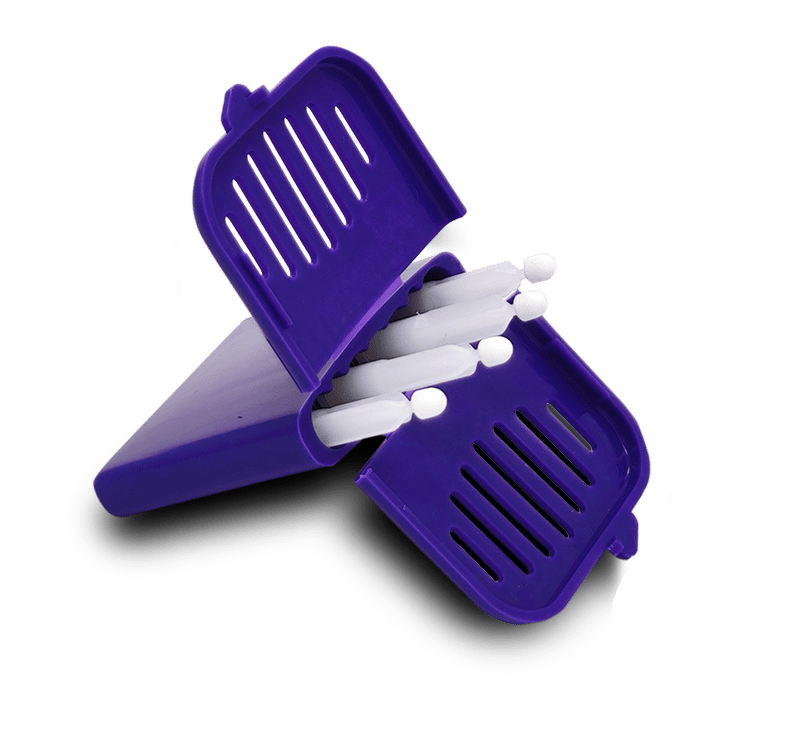
Mitra devices deliver tremendous ease & value to specimen collection!
Mitra® Microsampling Devices offer a streamlined, flexible, and decentralized approach to biological specimen collection. Enjoy the benefits of easier trial recruitment, increased adherence / compliance, elimination of cold-chain shipping / storage & reduced reliance on trained staff.

Build Your Own Kits or Sample Large Cohorts Remotely
With this format you can collect many samples in the field and ship them back to the lab in bulk. You may prefer to insert them into your own all-in-one kit.
- 10, 20, & 30 µL volumes
- Protective outer casing
- Barcoding to manage chain of custody
- Packed in specimen bag with desiccant for transport & storage

All-in-One Solution for Remote Specimen Collection
Our Mitra devices can be packed in convenient kits with sterile components for safe sample collection. Kits can be shipped to your end-users.
- 10, 20, & 30 µL volumes
- Protective outer casing
- Barcoding to manage chain of custody
- Packed in specimen bag with desiccant for transport & storage
- Contains devices & supplies
- Includes instructions for use & return shipping envelope
- Sized for easy global mailing

Accession & Process Multiple Mitra Samples at Once
This high-throughput solution allows you to rapidly process Mitra microsamples for method development, plate building, extractions - or all of the above!
- Racks available empty or filled with 96 microsamplers (10, 20, & 30 µL volumes)
- Filled racks come with a 2mL deep well storage plate
- Compatible with 96-channel pipettors & liquid-handling workstations
- Options with & without barcoding
Frequently Asked Questions
The Mitra device is not sold as a sterile product.
Evaluation devices are available on a case-by-case basis. A Microsampling Specialist will review the options with you during your initial project meeting.
Dried specimen Mitra samples are compatible with common lab instrumentation, including but not limited to liquid-handling workstations, LC-MS/MS, ELISA, Multi-Plex Immunoassays, and other analyzers.
The Mitra device does not contain any stabilizing agents.
Yes, we can customize the Mitra device formats and collection kits to your needs. This may include additional barcodes, custom instructions, study labels, or other details specific to your study or project. Please ask about available customizations during your meeting with a Microsampling Specialist.
Our microsampling devices are produced at Trajan's Center of Excellence in Malaysia and shipped to you from our regional distribution centers in the USA, Australia and the United Kingdom. Shipment lead times are dependent upon order volume but, typically, orders ship within 7-10 business days after we receive your purchase order. We encourage you to follow up with your Microsampling Specialist to help you track your order through the shipment process.
Mitra® devices are intended as specimen collectors and for the storage and transport of blood. They are CE-IVD in the UK and EU, a Class 1 IVD in Australia, Brazil & China, Class B in South Africa, and registered with health agencies in Canada and Ukraine. In the USA, Mitra devices are supplied as a Research Use Only (RUO) product to assist in method development, other research-related and non-diagnostic activities. Mitra has not been validated for use with any diagnostic testing. The suitability of Mitra for any analytical application must be evaluated and validated by the laboratory or research institute in a manner consistent with local regulatory requirements.
Our standard collection kit includes a Mitra device packed in a specimen bag with a sachet of drying desiccant, instructions for use, other supplies needed for safe remote sample collection, and a return envelope for mailing samples to the lab. Ask your Microsampling Specialist how kits may be customized per your project needs.
There are three options to purchase Mitra devices. Depending on your geographic locations, you can pay by credit card, invoice or proforma invoice (prepay by wire transfer).
We sell direct to most countries globally with the exception of a few countries, where we work with authorized distributors. Please contact us for country-specific details.
VAMS technology allows convenient at-home monitoring and is minimally invasive. It also offers efficiencies in research settings."
Dr. Christophe Stove,
Ghent University, Belgium
Mitra with VAMS technology offers simplicity and rapidity of use, while maintaining high performance and guaranteeing reliable data."
Dr. Laura Mercolini,
University of Bologna, Italy
“With a small fingerprick, study volunteers can use the Mitra devices to help scientists fight COVID-19 from their homes."
Dr. Kaitlyn Sadtler,
National Institutes of Health (NIH), US









Have more questions about pricing or need additional information?
Complete the form below and an experienced Microsampling Specialist will guide you through the Mitra device selection process in order to tailor the format/pricing to your unique project needs and budget.
