enhancing cosmeceutical product development through innovative Microbiopsy sampling


enhancing cosmeceutical product development through innovative Microbiopsy sampling
Nov 12, 2024 7:54:51 AM
3
min read
exploring alternatives to scalpel and traditional punch skin biopsies
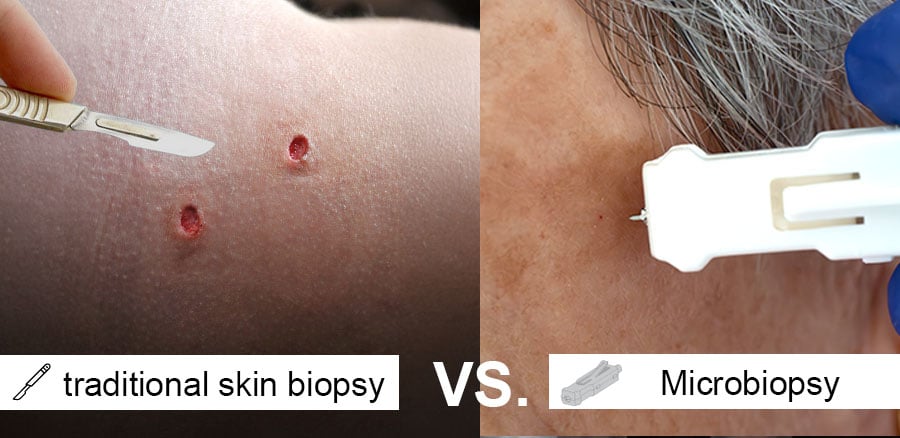

exploring alternatives to scalpel and traditional punch skin biopsies
Oct 30, 2024 1:22:52 PM
4
min read
the role of skin biopsy in infectious skin diseases research
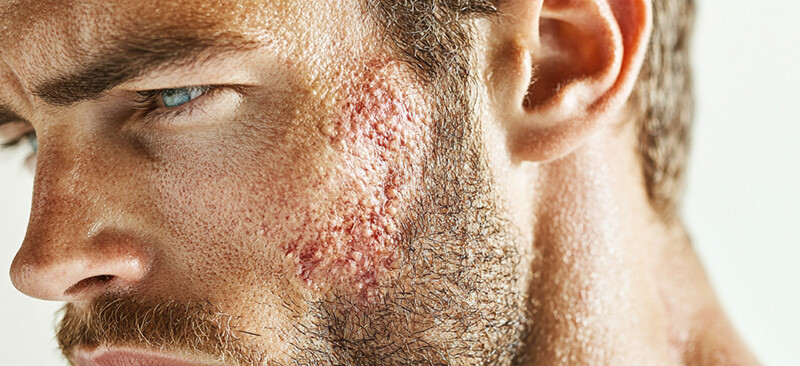

the role of skin biopsy in infectious skin diseases research
Oct 30, 2024 1:10:13 PM
4
min read
voices from the field: exploring Harpera’s global impact on dermatological research
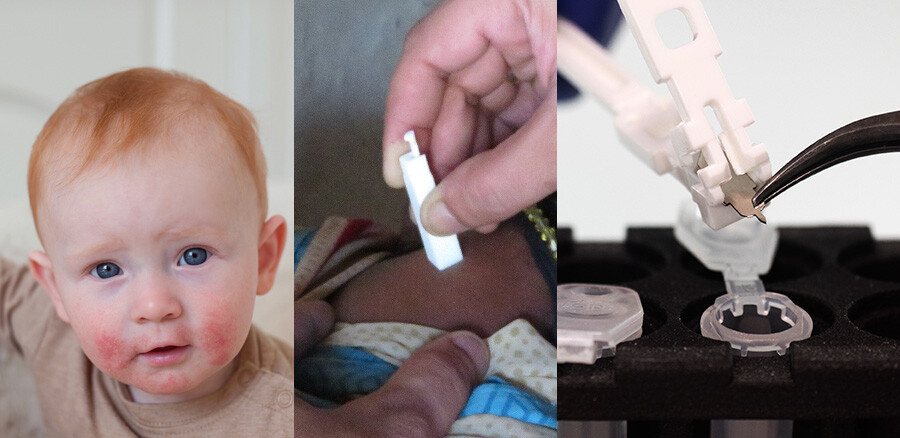

voices from the field: exploring Harpera’s global impact on dermatological research
Oct 10, 2024 1:49:10 PM
2
min read
microbiopsy punch may aid molecular profiling in dermatology
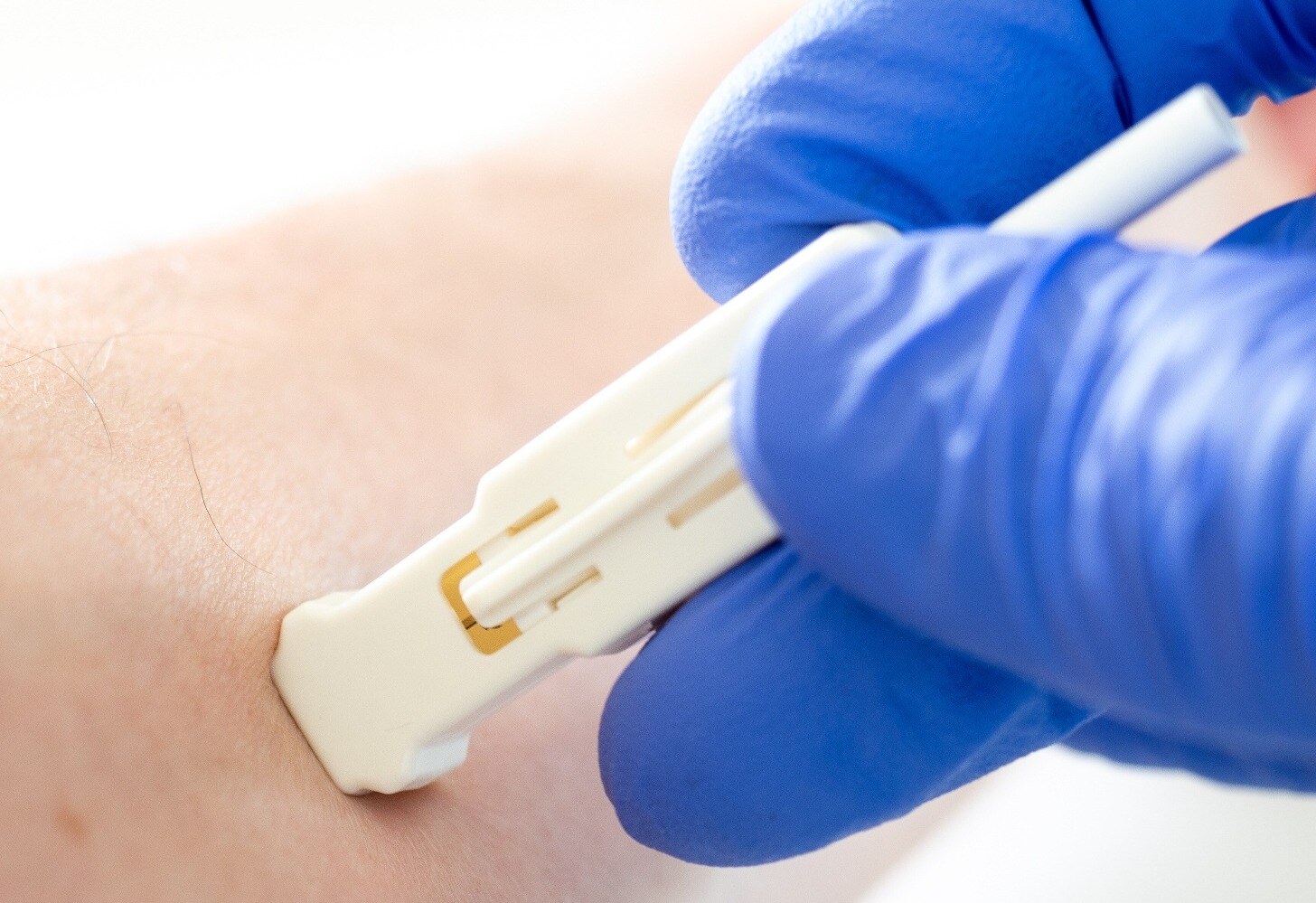

microbiopsy punch may aid molecular profiling in dermatology
Sep 20, 2024 10:29:00 AM
2
min read
urinary albumin creatinine ratio in dried microsamples


urinary albumin creatinine ratio in dried microsamples
Jun 3, 2024 9:00:00 AM
5
min read
