Share this
methotrexate TDM using whole blood microsamples
by James Rudge, PhD, Technical Director, Trajan on Aug 15, 2022 9:00:00 AM
An article by Dala N. Daraghmeh et al in Australia, published in the July 2022 issue of Analytical and Bioanalytical Chemistry, reported on an investigation into relative levels of methotrexate and methotrexate polyglutamate in different blood matrices.
The paper is entitled “Quantitation of methotrexate polyglutamates in human whole blood, erythrocytes and leukocytes collected via venepuncture and volumetric absorptive micro-sampling: a green LC–MS/MS-based method.” It describes a thorough investigation into the possibility of using whole blood sampling rather than plasma or washed red blood cells for remote therapeutic drug monitoring (TDM) of methotrexate.
The group concluded that using whole blood, collected via either venipuncture or remote Mitra® devices based on VAMS® technology, could save time while maintaining accuracy for measuring methotrexate and its metabolites.
What is Methotrexate?
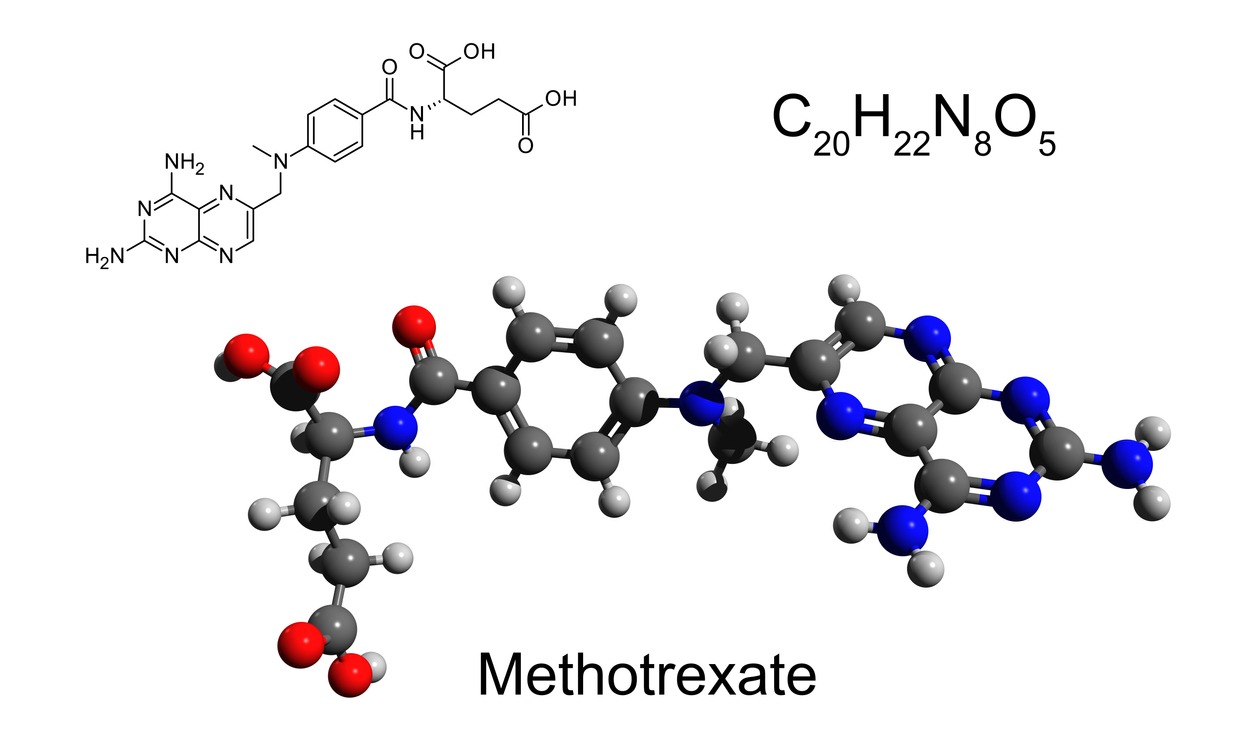 In 1948 Sidney Farber reported on the use of aminopterin, an amino derivative of folic acid, as an antineoplastic agent for the treatment of pediatric leukemia. During the ensuing years, it was noticed that the drug led to an interference in the proliferation of connective tissue. In 1951, a study showed improvements in patients with rheumatoid arthritis (RA), psoriasis and psoriatic arthritis [PSA) when they were given aminopterin. Due to issues with manufacturing, the drug was later modified with the addition of a methyl group.
In 1948 Sidney Farber reported on the use of aminopterin, an amino derivative of folic acid, as an antineoplastic agent for the treatment of pediatric leukemia. During the ensuing years, it was noticed that the drug led to an interference in the proliferation of connective tissue. In 1951, a study showed improvements in patients with rheumatoid arthritis (RA), psoriasis and psoriatic arthritis [PSA) when they were given aminopterin. Due to issues with manufacturing, the drug was later modified with the addition of a methyl group.
The resultant drug, methotrexate (MTX), was approved by the US Food and Drug Administration (FDA) in 1953 for the treatment of a number of conditions, including acute lymphoblastic leukemia (in combination with other therapies). Several studies conducted in the ensuing years also showed positive results for those suffering from PSA and RA.
As a result, in the 1980s, there was a widespread uptake in the use of MTX for treating diseases such as RA. There are several proposed mechanisms of action for MTX, including the inhibition of a number of enzymes, such as methylenetetrahydrofolate reductase, which can lead to an interference in DNA synthesis.
How Methotrexate Treats Rheumatoid Arthritis
 Rheumatoid Arthritis (RA) is an autoimmune disease which causes pain and swelling in joints, eventually leading to immobility. It was reported between 1980 and 2019 that the global prevalence of the disease was an average of 460 per 100,000 population.
Rheumatoid Arthritis (RA) is an autoimmune disease which causes pain and swelling in joints, eventually leading to immobility. It was reported between 1980 and 2019 that the global prevalence of the disease was an average of 460 per 100,000 population.
Methotrexate taken in low doses is an effective treatment for many, but it has been reported that 40% of RA patients do not respond well and others suffer from adverse side effects. It has also been reported that plasma-based therapeutic drug monitoring (TDM), can be useful for determining patient adherence.
However, the half-life of MTX in plasma ranges from 4.5 to 10 hours, leading to challenges in reliably measuring the drug concentration. When MTX enters blood cells, it is enzymatically converted into five MTX-polyglutamate metabolites (MTX-PGs), some of which are more pharmacologically active than the parent compound and also have longer half-lives (weeks to months). As a result, MTX-PGs have the potential to be a more reliable MTX response marker.
The site of action for MTX-PGs is thought to be lymphocytes, found as part of peripheral blood mononuclear cells (PBMCs). However, MTX-PGs also accumulate in red blood cells (RBCs) and in these cells have shown to have a half-life of 120 days.
As a result, RBCs have been considered surrogate cell types to measure MTX-PGs rather than PBMCs. However, it must be noted that MTX-PGs in RBCs may not be at the same concentration as found in lymphocytes, so may not be fully representative of the PBMC fraction.
Researching Improved Monitoring of Methotrexate
Given that it is time consuming to prepare washed RBCs or isolate PBMCs for MTX-PG measurement, the research group conducting the study (summarized here) wanted to compare concentrations of MTX and MTX-PGs in plasma, RBCS, venous whole blood and capillary whole blood (dried Mitra samples).
They hoped that measuring MTX and MTX-PGs in whole blood would simplify sample collection and analysis. They also hoped that using VAMS would allow for home sampling (as long as correlative data proved this in the study).
Methotrexate Study Method and Findings
- The group successfully developed and validated a high sensitivity HILIC LC-MS/MS for all matrices tested (in accordance with FDA guidelines).
- The group isolated PBMC, RBCs, plasma for extraction and analysis; wet and dried (20 µL Mitra) whole blood was also extracted and analyzed.
- Greenness evaluations for each method were scored using Green Analytical Procedure (GAPI) and an Analytical GREEnness Metric Approach (AGREE) tools.
- The Mitra-VAMS samples were stable at 37° C and room temperature for two weeks. The whole blood samples were stable at room temperature for 24h.
- When comparing MTX-PGs in patient samples at different timepoints, the distribution was found to be different in RBCs compared to PBMCs. This was due, in part, to the presence and absence of certain cellular proteins (RFC, GGH and FPG) that are involved with the transport of MTX in and out of cells. These proteins are not present in mature RBCs but are found in PBMCs.
- The group concluded that the concentration of MTX-PGs in PBMCs would be likely to be more dynamic compared to RBCs. Interestingly, immature RBCs do contain the protein RFC (which is involved in the uptake of MTX into the intracellular space).
- The team then evaluated the distribution of individual MTX-PGs and average concentrations of these in: washed RBCs, plasma, wet whole blood, and dried capillary Mitra-VAMS samples.
- They found that for plasma, only MTX-PG1 and MTX-PG2 was detectable, however, all 5 MTX-PGs (MTX-PG1-5) were seen in the other matrices tested.
- Total concentration of MTX-PG in washed RBCs was then compared to wet whole blood and VAMS. Mean ratio of calculated concentrations of 0.91–1.0 was seen for RBC:whole blood, whole blood: VAMS and RBC:VAMS.
- It was noted on initial dosing that the MTX parent would be artificially higher in whole blood / VAMS samples, compared to washed RBCs. This was due to incomplete clearance of the MTX parent in the plasma fraction after initial dosing.
- The group thus concluded that this would limit TDM collection in these matrices to, just before a dosing rather after dosing, as the whole blood matrices contained plasma as well as cells.
- In terms of the greenness of each method, VAMS was found to be the greenest with AGREE scores of 0.68 compared to 0.61 for RBC method.
Study Authors’ Conclusions
- Traditionally RBCs have been used as surrogates for PBMCs (thought to be site of action of MTX), but the group saw differences between the two matrices and would need to conduct more work to understand the implications of this.
- They reported that Mitra-VAMS microsample collection proved to be a greatly simplified and inexpensive method. They reported that the capillary microsampling method saves time, and that VAMS is ideal for self-monitoring as well as increasing self-awareness of medications. They commented that this would allow for a collaborative approach to care between physician and patient.
- It must be noted that the samples must be collected before the next dose of MTX for whole blood measurement, compared to washed WBCs.
- Understanding the metabolite accumulation in different blood fractions has the potential to understand MTX pharmacokinetics to allow for modifications of drug dose given.
Neoteryx Comments
Conducting bridging studies between VAMs extracts vs. traditional plasma processing is always recommended to understand if related biases are seen. However, the distribution of drug and/or metabolites may also be different, dependent on the site of action. This study illustrates the importance of knowing the pharmacodynamic nature of drug and metabolites, especially when the drug target is found in the blood.
This article was summarized for our readers by James Rudge, PhD, Neoteryx Technical Director. This is curated content. To learn more about the important research outlined in this blog, visit the original article in Analytical and Bioanalytical Chemistry.
You can access this microsampling article and others in our Technical Resource Library.
Image Credits: iStock, Neoteryx, Trajan Scientific and Medical
Share this
- Microsampling (206)
- Research, Remote Research (119)
- Venipuncture Alternative (105)
- Clinical Trials, Clinical Research (83)
- Mitra® Device (73)
- Therapeutic Drug Monitoring, TDM (51)
- Dried Blood Spot, DBS (39)
- Biomonitoring, Health, Wellness (30)
- Infectious Disease, Vaccines, COVID-19 (24)
- Blood Microsampling, Serology (23)
- Omics, Multi-Omics (21)
- Decentralized Clinical Trial (DCT) (20)
- Specimen Collection (18)
- Toxicology, Doping, Drug/Alcohol Monitoring, PEth (17)
- Skin Microsampling, Microbiopsy (14)
- hemaPEN® Device (13)
- Preclinical Research, Animal Studies (12)
- Pharmaceuticals, Drug Development (9)
- Harpera Device (7)
- Industry News, Microsampling News (5)
- Antibodies, MAbs (3)
- Company Press Release, Product Press Release (3)
- Environmental Toxins, Exposures (1)
- July 2025 (1)
- May 2025 (1)
- April 2025 (2)
- December 2024 (2)
- November 2024 (1)
- October 2024 (3)
- September 2024 (1)
- June 2024 (1)
- May 2024 (1)
- April 2024 (4)
- March 2024 (1)
- February 2024 (2)
- January 2024 (4)
- December 2023 (3)
- November 2023 (3)
- October 2023 (3)
- September 2023 (3)
- July 2023 (3)
- June 2023 (2)
- April 2023 (2)
- March 2023 (2)
- February 2023 (2)
- January 2023 (3)
- December 2022 (2)
- November 2022 (3)
- October 2022 (4)
- September 2022 (3)
- August 2022 (5)
- July 2022 (2)
- June 2022 (2)
- May 2022 (4)
- April 2022 (3)
- March 2022 (3)
- February 2022 (4)
- January 2022 (5)
- December 2021 (3)
- November 2021 (5)
- October 2021 (3)
- September 2021 (3)
- August 2021 (4)
- July 2021 (4)
- June 2021 (4)
- May 2021 (4)
- April 2021 (3)
- March 2021 (5)
- February 2021 (4)
- January 2021 (4)
- December 2020 (3)
- November 2020 (5)
- October 2020 (4)
- September 2020 (3)
- August 2020 (3)
- July 2020 (6)
- June 2020 (4)
- May 2020 (4)
- April 2020 (3)
- March 2020 (6)
- February 2020 (3)
- January 2020 (4)
- December 2019 (5)
- November 2019 (4)
- October 2019 (2)
- September 2019 (4)
- August 2019 (4)
- July 2019 (3)
- June 2019 (7)
- May 2019 (6)
- April 2019 (5)
- March 2019 (6)
- February 2019 (5)
- January 2019 (8)
- December 2018 (3)
- November 2018 (4)
- October 2018 (7)
- September 2018 (6)
- August 2018 (5)
- July 2018 (8)
- June 2018 (6)
- May 2018 (5)
- April 2018 (6)
- March 2018 (4)
- February 2018 (6)
- January 2018 (4)
- December 2017 (2)
- November 2017 (3)
- October 2017 (2)
- September 2017 (4)
- August 2017 (2)
- July 2017 (4)
- June 2017 (5)
- May 2017 (6)
- April 2017 (6)
- March 2017 (5)
- February 2017 (4)
- January 2017 (1)
- July 2016 (3)
- May 2016 (1)
- April 2016 (2)



Comments (1)