Share this
how stable are analytes in dried blood?
by James Rudge, PhD, Technical Director, Trajan on Dec 11, 2023 9:00:00 AM
One of the great benefits of dried blood microsampling is the fact that these samples can be remotely collected almost anywhere, at almost any time and by almost anyone. However, one of the concerns facing remote microsampling, is ensuring that the molecular integrity of the sample will remain intact during the shipping process.
The Challenge with Transporting Blood Samples
Do dried blood samples remain stable during shipping?
Degradation or extraction efficiency?
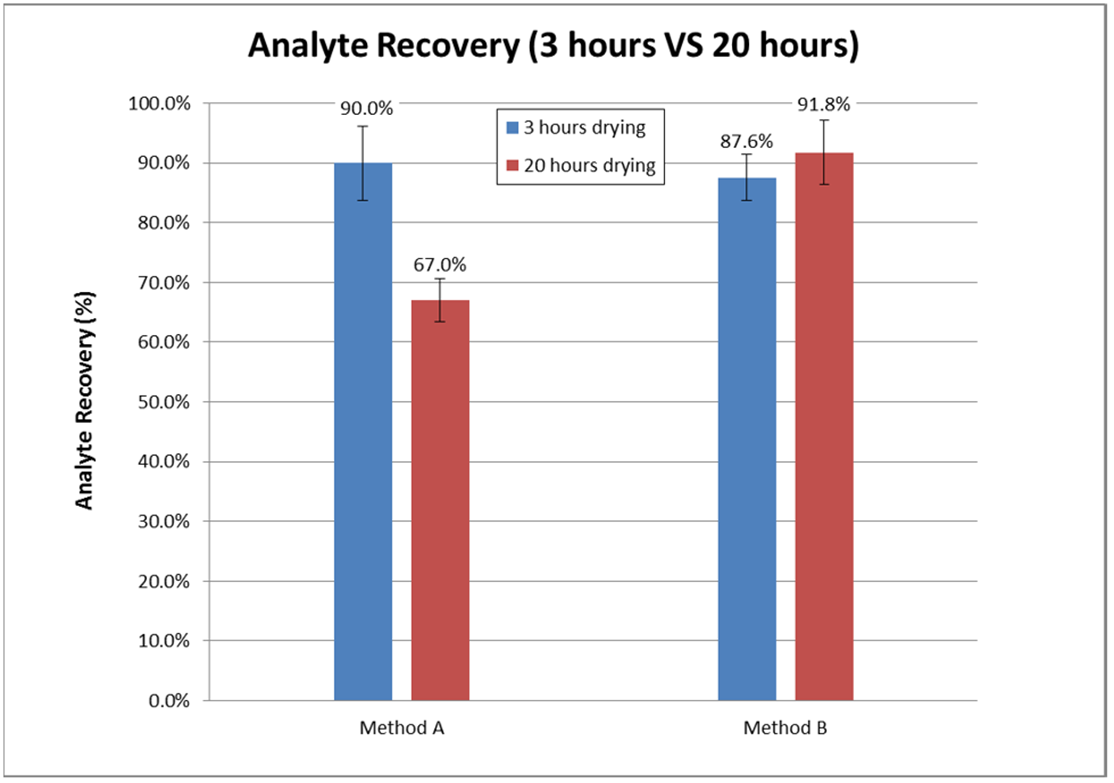
Figure 1. Recovery of corticosteroids under two different extraction conditions (Method B has a longer vortexing time).
Different microsampling device chemistries?
Sample Stability and the Journey of the Sample
Understanding Analyte Stability in Dried Blood Microsamples
With hundreds of peer-reviewed publications in the Microsampling Resource Library, researchers across disciplines have validated dried blood microsampling as a robust method for diverse analytes.
Learn More About Microsampling Advantages
If you are exploring stability, usability, or the broader scientific value of microsampling, we recommend visiting our in-depth page, Microsampling Capillary Blood Advantages. This page provides a clear overview of the technology, advantages, challenges, and real-world applications across clinical trials, pediatrics, TDM, population studies, and more.

Image Credits: iStock Photos, Trajan, Neoteryx
Share this
- Microsampling (206)
- Research, Remote Research (119)
- Venipuncture Alternative (105)
- Clinical Trials, Clinical Research (83)
- Mitra® Device (73)
- Therapeutic Drug Monitoring, TDM (51)
- Dried Blood Spot, DBS (39)
- Biomonitoring, Health, Wellness (30)
- Infectious Disease, Vaccines, COVID-19 (24)
- Blood Microsampling, Serology (23)
- Omics, Multi-Omics (21)
- Decentralized Clinical Trial (DCT) (20)
- Specimen Collection (18)
- Toxicology, Doping, Drug/Alcohol Monitoring, PEth (17)
- Skin Microsampling, Microbiopsy (14)
- hemaPEN® Device (13)
- Preclinical Research, Animal Studies (12)
- Pharmaceuticals, Drug Development (9)
- Harpera Device (7)
- Industry News, Microsampling News (5)
- Antibodies, MAbs (3)
- Company Press Release, Product Press Release (3)
- Environmental Toxins, Exposures (1)
- July 2025 (1)
- May 2025 (1)
- April 2025 (2)
- December 2024 (2)
- November 2024 (1)
- October 2024 (3)
- September 2024 (1)
- June 2024 (1)
- May 2024 (1)
- April 2024 (4)
- March 2024 (1)
- February 2024 (2)
- January 2024 (4)
- December 2023 (3)
- November 2023 (3)
- October 2023 (3)
- September 2023 (3)
- July 2023 (3)
- June 2023 (2)
- April 2023 (2)
- March 2023 (2)
- February 2023 (2)
- January 2023 (3)
- December 2022 (2)
- November 2022 (3)
- October 2022 (4)
- September 2022 (3)
- August 2022 (5)
- July 2022 (2)
- June 2022 (2)
- May 2022 (4)
- April 2022 (3)
- March 2022 (3)
- February 2022 (4)
- January 2022 (5)
- December 2021 (3)
- November 2021 (5)
- October 2021 (3)
- September 2021 (3)
- August 2021 (4)
- July 2021 (4)
- June 2021 (4)
- May 2021 (4)
- April 2021 (3)
- March 2021 (5)
- February 2021 (4)
- January 2021 (4)
- December 2020 (3)
- November 2020 (5)
- October 2020 (4)
- September 2020 (3)
- August 2020 (3)
- July 2020 (6)
- June 2020 (4)
- May 2020 (4)
- April 2020 (3)
- March 2020 (6)
- February 2020 (3)
- January 2020 (4)
- December 2019 (5)
- November 2019 (4)
- October 2019 (2)
- September 2019 (4)
- August 2019 (4)
- July 2019 (3)
- June 2019 (7)
- May 2019 (6)
- April 2019 (5)
- March 2019 (6)
- February 2019 (5)
- January 2019 (8)
- December 2018 (3)
- November 2018 (4)
- October 2018 (7)
- September 2018 (6)
- August 2018 (5)
- July 2018 (8)
- June 2018 (6)
- May 2018 (5)
- April 2018 (6)
- March 2018 (4)
- February 2018 (6)
- January 2018 (4)
- December 2017 (2)
- November 2017 (3)
- October 2017 (2)
- September 2017 (4)
- August 2017 (2)
- July 2017 (4)
- June 2017 (5)
- May 2017 (6)
- April 2017 (6)
- March 2017 (5)
- February 2017 (4)
- January 2017 (1)
- July 2016 (3)
- May 2016 (1)
- April 2016 (2)


No Comments Yet
Let us know what you think