Trajan appoints AppMed Inc. as Canadian distributor for Microsampling technologies, featuring Mitra® and Harpera®


Trajan appoints AppMed Inc. as Canadian distributor for Microsampling technologies, featuring Mitra® and Harpera®
December 3,2025
3
min read
Neoteryx and Cellecta partner to introduce innovative finger-prick blood test for comprehensive whole-genome expression profiling
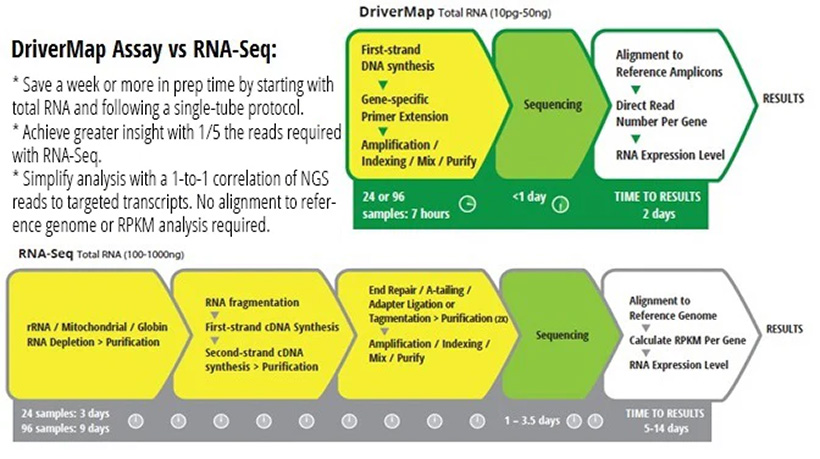

Neoteryx and Cellecta partner to introduce innovative finger-prick blood test for comprehensive whole-genome expression profiling
May 14,2025
2
min read
Psoriatic Arthritis Study Raises Hope for Earlier Intervention


Psoriatic Arthritis Study Raises Hope for Earlier Intervention
May 21,2024
3
min read
Eurofins Japan Offers PFAS Testing via Microsampling


Eurofins Japan Offers PFAS Testing via Microsampling
December 18,2023
3
min read
Trajan Distributes RNA Stabilization Solution for Mitra Microsampling


Trajan Distributes RNA Stabilization Solution for Mitra Microsampling
November 28,2023
3
min read
Bioanalytic to Distribute Trajan's Microsampling Devices in Poland


Bioanalytic to Distribute Trajan's Microsampling Devices in Poland
November 27,2023
3
min read
