Share this
Neoteryx Tailors Mitra Collection Kits for Kids
by Neoteryx Microsampling on December 1,2021
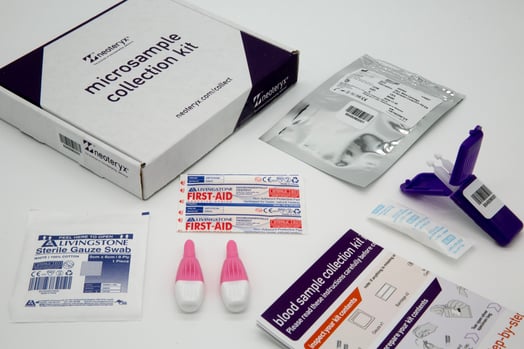
December 2021 — US-based medical device company Neoteryx announces its user-friendly at-home specimen collection kit for children in support of labs and research customers that conduct pediatric studies, clinical trials and children’s health programs around the world. The pediatric version is a modification of the company’s popular Mitra® Sample Collection Kit to now include contact-activated medium flow BD Microtainer® Pink Lancets with a fine-gauge needle (21 G x 1.8mm depth) for a gentler finger-prick approach to blood collection. The pink lancets are intended for collecting samples from kids aged one year and older, as well as from older adults who have delicate skin.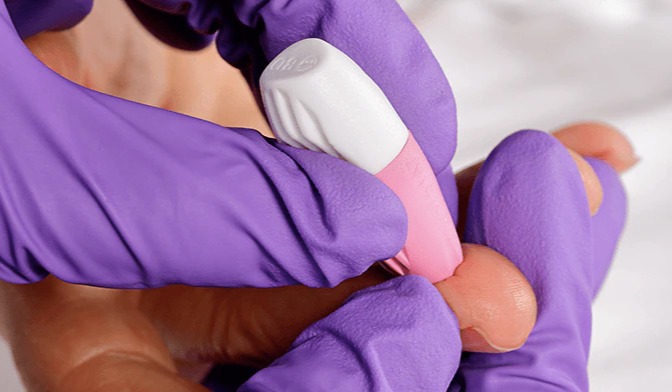
“Our customers conducting research in children and other vulnerable populations expressed a wish to ensure a nearly painless sampling experience for their study subjects,” said James Rudge, PhD, Technical Director, Neoteryx. “Pediatric researchers can now purchase Mitra® Sample Collection Kits for children’s studies that include pink lancets to enable delivery of a gentler sample collection experience.”
 Modifying the Mitra Sample Collection Kits with pink lancets may also be suitable for study subjects who experience needle anxiety or stress during blood collection procedures.
Modifying the Mitra Sample Collection Kits with pink lancets may also be suitable for study subjects who experience needle anxiety or stress during blood collection procedures.
“People may feel more at ease knowing that the pink lancets feature a tiny, delicate needle, yet when paired with Mitra devices that have 10µL and 20µL absorbent VAMS® tips, they still produce a medium flow that is sufficient to collect good sample volumes,” Dr. Rudge added.
Where greater blood volume is needed for certain studies, the traditional Mitra Sample Collection Kit containing the high-flow BD Microtainer Blue Lancet (1.5mm blade x 2.0mm depth) will work best with Mitra devices that have 30µL VAMS tips. Both traditional and pediatric Mitra Sample Collection Kits from Neoteryx include multiple lancets inside the kit box. Typically, only 1 finger-stick is needed to collect enough blood to fill a Mitra device, but the extra lancets are provided as a back-up measure.
The company extends its gratitude to the many collaborative customers who have shared their experiences about using Neoteryx's volumetric absorptive microsampling products in their work with pediatric study subjects and families. Real-world feedback helps the company advance its product offerings to meet the needs of the broader microsampling community.
Customers who wish to order Mitra Sample Collection Kits tailored for pediatric studies can reference Part Number 200505: 20uL 2-Sampler Cartridge Collection Kit with BD Pink Lancets. If a customer is working with newborns or infants younger than 12 months or needs additional components in their kits or other customizations, they can reach out to a Microsampling Specialist at Neoteryx for more information: info@neoteryx.com.
About Neoteryx
Neoteryx LLC, a medical device company in Southern California, delivers simple, quantitative and automatable microsampling solutions. Its Mitra® device facilitates remote specimen collection and transport of blood and other biological fluids to improve human health, reduce laboratory costs and enable new models of care. Neoteryx’s customers include scientific researchers, laboratories and health providers working to advance telemedicine, pharmaceutical development, biotechnology research and clinical diagnostics. For more information, visit www.neoteryx.com.
Mitra devices are intended as a specimen collector and for the storage and transport of biological fluids. They are CE-IVD self-certified in the UK and EU, a Class 1 IVD in Australia, Brazil and China, a Class B in South Africa, and registered with health agencies in Canada, Thailand, and Ukraine. In the United States, Mitra devices are for Research Use Only (RUO). In some countries, Mitra devices may be used in clinical diagnostic laboratory systems after the laboratory has validated their complete system in compliance with relevant rules and regulations. Neoteryx operates a Quality Management System (QMS) that is based on FDA good manufacturing practices, 21 CFR 820 regulations, and ISO-13485.
Image Credits: BD Microtainer images courtesy of BD (www.bd.com), Neoteryx

Share this
- Microsampling (41)
- Industry News, Microsampling News (37)
- Mitra® Device (34)
- Company Press Release, Product Press Release (22)
- Research, Remote Research (18)
- Infectious Disease, Vaccines, COVID-19 (15)
- Clinical Trials, Clinical Research (14)
- Biomonitoring, Health, Wellness (10)
- Blood Microsampling, Serology (10)
- Decentralized Clinical Trial (DCT) (8)
- Omics, Multi-Omics (7)
- Venipuncture Alternative (6)
- Skin Microsampling, Microbiopsy (5)
- Harpera Device (3)
- Specimen Collection (3)
- Toxicology, Doping, Drug/Alcohol Monitoring, PEth (3)
- Pharmaceuticals, Drug Development (2)
- Therapeutic Drug Monitoring, TDM (2)
- Antibodies, MAbs (1)
- Environmental Toxins, Exposures (1)
- Preclinical Research, Animal Studies (1)
- hemaPEN® Device (1)
- February 2026 (1)
- January 2026 (1)
- December 2025 (1)
- May 2025 (1)
- April 2025 (1)
- February 2025 (4)
- September 2024 (1)
- August 2024 (1)
- May 2024 (2)
- January 2024 (1)
- December 2023 (2)
- November 2023 (3)
- October 2023 (2)
- September 2023 (1)
- August 2023 (3)
- July 2023 (3)
- June 2023 (1)
- May 2023 (2)
- April 2023 (1)
- March 2023 (2)
- February 2023 (1)
- January 2023 (2)
- December 2022 (1)
- November 2022 (1)
- October 2022 (2)
- August 2022 (1)
- April 2022 (1)
- February 2022 (1)
- January 2022 (1)
- December 2021 (1)
- November 2021 (1)
- October 2021 (2)
- September 2021 (1)
- August 2021 (2)
- July 2021 (2)
- June 2021 (2)
- April 2021 (1)
- March 2021 (2)
- February 2021 (1)
- January 2021 (1)
- December 2020 (1)
- November 2020 (1)
- October 2020 (1)
- September 2020 (2)
- August 2020 (3)
- July 2020 (3)
- June 2020 (2)
- May 2020 (1)
- April 2020 (3)
- October 2019 (1)
- March 2019 (1)
- January 2019 (1)
- November 2018 (1)
- August 2018 (1)
- July 2018 (1)
- June 2017 (1)
- April 2017 (1)
- March 2017 (1)
- February 2017 (1)
- May 2016 (1)
- December 2015 (1)
- October 2015 (1)
- August 2015 (1)
- August 2014 (1)
- July 2014 (1)


Comments (2)