Share this
the use of Mitra for phase 2b/phase 3 clinical trials for padsevonil
by Neoteryx Microsampling on Jun 20, 2019 7:47:00 AM
The 2019 conference of the European Federation for Exploratory Medicines Development (EUFEMED), held May 15 – 17 in Lyon, France, turned a spotlight on “The Changing Landscape of Early Medicines Development.” This included a presentation by Hugues Chanteux on some exciting new work he’s done with his team at UCB Pharma, an innovative pharma company that has done much to move forward the science of blood microsampling.
The topic at hand was Padsevonil (PSL), a drug in development that shows promise for treating patients with epilepsy and drug-resistant focal seizures. At the time of writing, it was in Phase 2b / Phase 3 in clinical trials. Chanteaux explained how he and his team validated a method for the collection of PK samples in clinical studies of PSL using Mitra® microsampling devices.
Microsampling Advantages for Antiseizure Medication Studies
The advantages of using Mitra devices in clinical trials are many. This method of precise, low-volume specimen collection reduces the burden both on clinical trial participants and on clinical operations, as well as allowing for more flexibility in recruitment and participation by introducing more convenient at-home and remote sampling.
In his presentation, Chanteux walks through his team’s methodology and delivers its results.
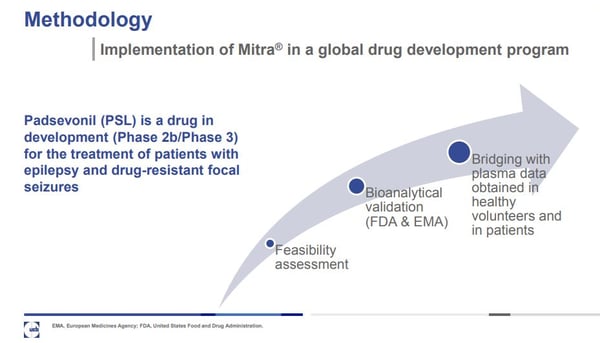
Bioanalytical Validation: The bioanalytical method was demonstrated to be accurate, precise, and selective for quantification of PSL over a clinically relevant concentration range (2-2000 ng/mL).
Bridging of Clinical Data: Blood PSL exposure was ~34% lower than plasma exposure This is in close agreement with the measured in vitro blood to plasma ratio of PSL (0.7).
With the bioanalytical method for quantification of PSL using microsampling successfully validated and PK data obtained with microsampling bridged with that from plasma, both in healthy study participants and patients with epilepsy, UCB Pharma has implemented the use of Mitra blood collection systems for collection of PK samples in global development studies.
For full results, see Hugues Chanteux’s presentation here. For more information on using Mitra devices and Volumetric Absorptive Microsampling technology in pharma, clinical trials, and drug development, view our full collection of relevant resources.

Share this
- Microsampling (206)
- Research, Remote Research (119)
- Venipuncture Alternative (105)
- Clinical Trials, Clinical Research (83)
- Mitra® Device (73)
- Therapeutic Drug Monitoring, TDM (51)
- Dried Blood Spot, DBS (39)
- Biomonitoring, Health, Wellness (30)
- Infectious Disease, Vaccines, COVID-19 (24)
- Blood Microsampling, Serology (23)
- Omics, Multi-Omics (21)
- Decentralized Clinical Trial (DCT) (20)
- Specimen Collection (18)
- Toxicology, Doping, Drug/Alcohol Monitoring, PEth (17)
- Skin Microsampling, Microbiopsy (14)
- hemaPEN® Device (13)
- Preclinical Research, Animal Studies (12)
- Pharmaceuticals, Drug Development (9)
- Harpera Device (7)
- Industry News, Microsampling News (5)
- Antibodies, MAbs (3)
- Company Press Release, Product Press Release (3)
- Environmental Toxins, Exposures (1)
- July 2025 (1)
- May 2025 (1)
- April 2025 (2)
- December 2024 (2)
- November 2024 (1)
- October 2024 (3)
- September 2024 (1)
- June 2024 (1)
- May 2024 (1)
- April 2024 (4)
- March 2024 (1)
- February 2024 (2)
- January 2024 (4)
- December 2023 (3)
- November 2023 (3)
- October 2023 (3)
- September 2023 (3)
- July 2023 (3)
- June 2023 (2)
- April 2023 (2)
- March 2023 (2)
- February 2023 (2)
- January 2023 (3)
- December 2022 (2)
- November 2022 (3)
- October 2022 (4)
- September 2022 (3)
- August 2022 (5)
- July 2022 (2)
- June 2022 (2)
- May 2022 (4)
- April 2022 (3)
- March 2022 (3)
- February 2022 (4)
- January 2022 (5)
- December 2021 (3)
- November 2021 (5)
- October 2021 (3)
- September 2021 (3)
- August 2021 (4)
- July 2021 (4)
- June 2021 (4)
- May 2021 (4)
- April 2021 (3)
- March 2021 (5)
- February 2021 (4)
- January 2021 (4)
- December 2020 (3)
- November 2020 (5)
- October 2020 (4)
- September 2020 (3)
- August 2020 (3)
- July 2020 (6)
- June 2020 (4)
- May 2020 (4)
- April 2020 (3)
- March 2020 (6)
- February 2020 (3)
- January 2020 (4)
- December 2019 (5)
- November 2019 (4)
- October 2019 (2)
- September 2019 (4)
- August 2019 (4)
- July 2019 (3)
- June 2019 (7)
- May 2019 (6)
- April 2019 (5)
- March 2019 (6)
- February 2019 (5)
- January 2019 (8)
- December 2018 (3)
- November 2018 (4)
- October 2018 (7)
- September 2018 (6)
- August 2018 (5)
- July 2018 (8)
- June 2018 (6)
- May 2018 (5)
- April 2018 (6)
- March 2018 (4)
- February 2018 (6)
- January 2018 (4)
- December 2017 (2)
- November 2017 (3)
- October 2017 (2)
- September 2017 (4)
- August 2017 (2)
- July 2017 (4)
- June 2017 (5)
- May 2017 (6)
- April 2017 (6)
- March 2017 (5)
- February 2017 (4)
- January 2017 (1)
- July 2016 (3)
- May 2016 (1)
- April 2016 (2)


Comments (1)