Share this
microsampling to measure mAbs in children with inflammatory bowel disease
by James Rudge, PhD, Technical Director, Trajan on Apr 26, 2022 10:04:51 AM
An article by Marieke Zijlstra et al at Sanguin in the Netherlands published in the January 2021 issue of the Journal of Pediatric Gastroenterology and Nutrition reports on a pilot bridging study of inflammatory bowel disease in children that compared capillary samples to paired venous sera.
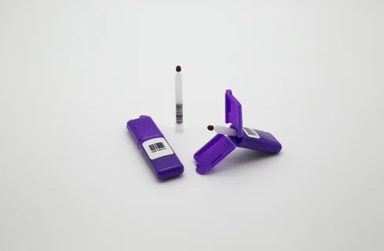 The paper is entitled “Infliximab Level Between Venous and Capillary Blood Using Novel Device Strongly Correlate in Paediatric Inflammatory Bowel Disease Patients.” It describes a small clinical study measuring infliximab (IFX) levels in both venous blood and capillary blood (collected on Mitra® devices based on VAMS® technology).
The paper is entitled “Infliximab Level Between Venous and Capillary Blood Using Novel Device Strongly Correlate in Paediatric Inflammatory Bowel Disease Patients.” It describes a small clinical study measuring infliximab (IFX) levels in both venous blood and capillary blood (collected on Mitra® devices based on VAMS® technology).
With this study, the research team was able to conclude that dried blood sampling using Mitra devices with VAMS was a good candidate for measuring IFX blood levels in pediatric patients and would facilitate home sampling for therapeutic drug monitoring (TDM).
Understanding Inflammatory Bowel Disease and mAb Therapies
Cytokines play a key role in inflammatory bowel disease (IBD). Tumor necrosis factor-alpha (TNFα) is a cytokine produced primarily from macrophages. Macrophages are immune cells that are an essential part of the innate immune system. TNFα plays a key role in a number of conditions such as lesions, tumor development, infection and inflammation.
When released from host macrophages, TNFα activates further inflammatory responses, such as the release of other proinflammatory cytokines. Moreover, TNFα has a direct effect on the intestinal epithelial barrier disrupting intestinal tight junctions. In doing so, the cytokine acts to increase inflammation of the gut and, in some cases, leads to inflammatory bowel disease (IBD). IBD is a term often used to describe two conditions: ulcerative colitis (large intestine only) and Crohn’s disease (which can affect any part of the digestive system.
There are a number of drugs with anti-TNFα activity, and these include infliximab (IFX) and adalimumab (ALD). Both drugs are monoclonal antibodies (mAbs), which act to neutralize the function of TNFα preventing it from binding to its receptors. Both drugs have been shown to be very effective in treating IBD in both adults and children.
Treating and Monitoring Pediatric Inflammatory Bowel Disease (PIBD)
For children, information on dosing of these mAb drugs is limited to extrapolating from adult data, which is not sufficient to provide accurate and individualized dosing. Indeed, it is critical to get the dose right for each individual, as low dose levels have been associated with a loss of therapeutic response. In their study paper, the authors quote a previous study where pediatric “patients with trough levels during maintenance treatment of 5 µg/mL [of IFX] are more likely to show a clinical response.”
Therapeutic drug monitoring (TDM) of children on IFX is currently conducted in clinic by venipuncture. To minimize the number of needle sticks, TDM is often done during the same appointment or time of the next administration of infliximab. This means that any dose adjustments must wait until the next drug infusion appointment, which could be every few weeks and is not ideal. Use of dried blood sampling can be performed more frequently and outside of the hospital, which could help with optimizing dosing and more individualized and timely administration of IFX.
The authors highlighted that dried blood samples (both VAMS and dried blood spots) had been previously demonstrated in adult cohorts to monitor IFX levels. An investigation of the application of dried blood sampling had not yet been demonstrated in children undergoing TDM of IFX. The aim of this study was to compare venipuncture to dried microsamples with pediatric inflammatory bowel disease (PIBD) patients.
Pediatric IBD Study Methods
- Recruitment of pediatric patients (n=20) included those with a diagnosis of Crohn’s disease, ulcerative colitis, or IBD unclassified; all were receiving IFX.
- Both venous blood (for serum) and capillary blood (on Mitra devices with VAMS) were collected simultaneously. To account for differences in IFX levels in whole blood to serum, a mean hematocrit (HCT) value of 0.42 was assumed and a correction factor applied to the VAMS extracts. Moreover, each venous sample was measured for its individual HCT for comparison and also for comparison to the fixed HCT value (0.42).
- Both IFX and anti-IFX levels were analyzed using ELISA assays.
- The LLOQ for IFX was 0.6 µg/mL for VAMS and 0.03 µg/mL for serum; the difference was due to the different dilution of the two matrices.
- An antigen binding test was also conducted to measure the anti-IFX raised antibodies; the LLOQ and ULOQ were the same for both serum and VAMS.
- Bland-Altman, ICC, Cohen Kappa and Spearman approaches were used for statistical analysis.
Study Authors’ Results and Discussion
- Out of 20 pediatric patients, four had IFX levels below the LLOQ for the dried samples and 1 below the LLOQ for the serum samples.
- In one patient, antidrug antibodies were also detected.
- Strong correlations were seen comparing dried VAMS samples to serum samples (r = >0.989 P<0.001).
- The data for 0.42 HCT correction and per-patient HCT correction were almost identical to each other.
- From the Bland-Altman calculation, there was no significant difference observed between the serum and VAMS samples (-0.14 (95%[CI]: 0.44; 0.16).
- Using ICC, interrater reliability was 0.998; 95% CI: 0.995–0.999.
- The IFX trough levels measuring 3 clinically relevant categories (low, adequate, and high) using the Cohen Kappa method showed excellent values of 0.911 (P=0.0001).
- The study shows that the dried sampling with VAMS approach “is a reliable method to measure IFX levels in children allowing “patients to perform the blood sampling at home, which would greatly simplify TDM.” Furthermore, two previous studies have shown that more frequent sampling events improved IFX durability during the first year on the drug.
- The study authors commented that remote finger-stick sampling was less invasive and proposed that this approach would help medical centers that weren’t set up to measure IFX ‘in-house’.
- The study authors propose that additional benefits would be seen by combining IFX levels measured with remote sampling and using an IFX dosing mobile app.
- The group noted that the cost of dried sampling would be slightly higher than serum but that it could ultimately be more cost-effective because it would help to personalize IFX treatment, reduce repeat visits to clinic, potentially reduce relapse in IBD, and improve adherence to treatment.
- The group also highlighted another study in 40 adults with IBD who were receiving IFX. Strong correlations were between VAMS samples compared to serum samples. Other studies showed good correlations with dried blood spot measuring ADL. The researchers concluded that independent of "the type of TNF inhibitor and microsampling device, the finger prick is reliable to measure concentrations of the biologicals."
- Fixed HCT values can be used to correct for IFX levels from Mitra devices and suggested that it was unnecessary to measure HCT at home, even when 5 patients had significant anemia (HCT < 0.36) but said that this should be interpreted with caution due to the small cohort used.
- The results of this pilot study can be used in future studies measuring PK and PD in pediatrics. This would potentially include patient satisfaction.
- The authors stated that their study showed that the microsampling technology was a good candidate to measure IFX blood levels in pediatric patients and thus could facilitate the possibility for home sampling in TDM.
Final Thoughts from Neoteryx
This work provides another example of the importance of bridging between capillary and venous blood. It also demonstrates the utility of home sampling to provide the potential for improving treatment by allowing for sampling in advance of IV administration of a drug rather than during the same appointment.
Furthermore, finger-stick sampling appears to be a less stressful specimen collection approach than venipuncture for pediatric patients and study subjects. Indeed, a few years ago, Christophe Stove’s group at Ghent University ran a survey on adults and children in their study where finger-stick microsampling with Mitra-VAMS devices were the preferred choice compared to venipuncture and dried blood spot (DBS).
In terms of HCT correction, it is interesting to see that both an average HCT and per person HCT correction gave an almost identical correction. However, I agree with the authors that this must be approached with caution, especially where cohorts are expected to have a wide HCT range.
To learn more about how microsampling is being applied in Therapeutic Drug Monitoring visit out Resources Page.
This study paper was summarized for our readers by James Rudge, PhD, Neoteryx Technical Director. This is curated content. To learn more about the important research outlined in this review, visit the original article published in the Journal of Pediatric Gastroenterology and Nutrition.
Image Credits: IBD, IBS image iStock, Neoteryx
Share this
- Microsampling (206)
- Research, Remote Research (119)
- Venipuncture Alternative (105)
- Clinical Trials, Clinical Research (83)
- Mitra® Device (73)
- Therapeutic Drug Monitoring, TDM (51)
- Dried Blood Spot, DBS (39)
- Biomonitoring, Health, Wellness (30)
- Infectious Disease, Vaccines, COVID-19 (24)
- Blood Microsampling, Serology (23)
- Omics, Multi-Omics (21)
- Decentralized Clinical Trial (DCT) (20)
- Specimen Collection (18)
- Toxicology, Doping, Drug/Alcohol Monitoring, PEth (17)
- Skin Microsampling, Microbiopsy (14)
- hemaPEN® Device (13)
- Preclinical Research, Animal Studies (12)
- Pharmaceuticals, Drug Development (9)
- Harpera Device (7)
- Industry News, Microsampling News (5)
- Antibodies, MAbs (3)
- Company Press Release, Product Press Release (3)
- Environmental Toxins, Exposures (1)
- July 2025 (1)
- May 2025 (1)
- April 2025 (2)
- December 2024 (2)
- November 2024 (1)
- October 2024 (3)
- September 2024 (1)
- June 2024 (1)
- May 2024 (1)
- April 2024 (4)
- March 2024 (1)
- February 2024 (2)
- January 2024 (4)
- December 2023 (3)
- November 2023 (3)
- October 2023 (3)
- September 2023 (3)
- July 2023 (3)
- June 2023 (2)
- April 2023 (2)
- March 2023 (2)
- February 2023 (2)
- January 2023 (3)
- December 2022 (2)
- November 2022 (3)
- October 2022 (4)
- September 2022 (3)
- August 2022 (5)
- July 2022 (2)
- June 2022 (2)
- May 2022 (4)
- April 2022 (3)
- March 2022 (3)
- February 2022 (4)
- January 2022 (5)
- December 2021 (3)
- November 2021 (5)
- October 2021 (3)
- September 2021 (3)
- August 2021 (4)
- July 2021 (4)
- June 2021 (4)
- May 2021 (4)
- April 2021 (3)
- March 2021 (5)
- February 2021 (4)
- January 2021 (4)
- December 2020 (3)
- November 2020 (5)
- October 2020 (4)
- September 2020 (3)
- August 2020 (3)
- July 2020 (6)
- June 2020 (4)
- May 2020 (4)
- April 2020 (3)
- March 2020 (6)
- February 2020 (3)
- January 2020 (4)
- December 2019 (5)
- November 2019 (4)
- October 2019 (2)
- September 2019 (4)
- August 2019 (4)
- July 2019 (3)
- June 2019 (7)
- May 2019 (6)
- April 2019 (5)
- March 2019 (6)
- February 2019 (5)
- January 2019 (8)
- December 2018 (3)
- November 2018 (4)
- October 2018 (7)
- September 2018 (6)
- August 2018 (5)
- July 2018 (8)
- June 2018 (6)
- May 2018 (5)
- April 2018 (6)
- March 2018 (4)
- February 2018 (6)
- January 2018 (4)
- December 2017 (2)
- November 2017 (3)
- October 2017 (2)
- September 2017 (4)
- August 2017 (2)
- July 2017 (4)
- June 2017 (5)
- May 2017 (6)
- April 2017 (6)
- March 2017 (5)
- February 2017 (4)
- January 2017 (1)
- July 2016 (3)
- May 2016 (1)
- April 2016 (2)



No Comments Yet
Let us know what you think