Share this
expert Q&A: Harpera used for skin microsampling in leishmaniasis study
by Florian Lapierre, PhD, Microsampling Product Director on Apr 3, 2023 9:00:00 AM
Trajan's Neoteryx microsampling product director, Florian Lapierre, PhD, and colleagues recently interviewed scientists at Charles University in Prague, Czech Republic, where researchers in the Laboratory of Vector Biology, Department of Parasitology is using our Harpera™ Microbiopsy™ Punch to study the skin disease leishmaniasis.
The lab, which was founded nearly 30 years ago by Professor Petr Volf, combines laboratory and field research on leishmaniasis and its research team has also worked in endemic countries like Ethiopia and Algeria. We spoke with Jovana Sádlová, PhD, Department of Parasitology, Faculty of Science at Charles University, who discussed their current project with us. Dr. Jovana Sádlová in Ethiopia, where she conducted field work as part of a study on leishmaniasis disease.
Dr. Jovana Sádlová in Ethiopia, where she conducted field work as part of a study on leishmaniasis disease.
Dr. Lapierre: Can you please explain the research you and the team at Charles University are conducting?
Dr. Sádlová: The subject of our research is leishmaniasis, the second most important vector-borne infectious disease after malaria. We are trying to gain an understanding of the leishmania epidemiology and develop accurate animal modelling of disease transmission.
 The causative agents, Leishmania, are unicellular flagellated parasites (Kinetoplastida: Trypanosomatidae), which are transmitted by tiny insects called phlebotomine sand flies (Diptera: Psychodidae).
The causative agents, Leishmania, are unicellular flagellated parasites (Kinetoplastida: Trypanosomatidae), which are transmitted by tiny insects called phlebotomine sand flies (Diptera: Psychodidae).
Parasites usually circulate as zoonoses among their reservoir hosts, and humans become infected accidentally. More than 20 species of leishmania cause human disease in this way.
In our lab, we have a collection of over 100 Leishmania strains and a worldwide unique set of 12 sand fly colonies. We also breed uncommon rodent species that we are investigating as potential reservoir and model hosts for Leishmania. All this enables us to study different aspects of Leishmania – sand fly – host interactions and transmission cycles.
We use various laboratory methods, from DNA and protein analysis, histology and electron microscopy to immunology and classical entomology. In addition to laboratory work, we also undertake field research in leishmaniasis endemic areas, mostly in countries around the Mediterranean.
Q: Can you provide some background on leishmaniasis and why your work is important?
A: Leishmaniasis is a skin disease that has endemic transmission reported in 98 countries on 5 continents, with incidence of 1-2 million cases per year. Leishmaniasis is one of the most important parasitic diseases in the world. According to the World Health Organization (WHO), about 12 million people suffer from it and 10-20 thousand people die from the disease every year.
There are several distinct types of leishmaniasis. If the parasite causes exclusively cutaneous ulcerations, we speak of cutaneous leishmaniasis (CL). These ulcers often heal on their own, but permanent scars may remain in their place as a lifelong stigma. Some patients may develop more severe recurrent or diffuse CL that is difficult and time-consuming to treat.
Some Leishmania species can also cause mucosal lesions and cartilage destruction in the oronasal and pharyngeal regions. This chronic disease (mucocutaneous leishmaniasis) is not self-healing and can be fatal.
The most serious form of the disease is visceral leishmaniasis, also known as kala-azar (i.e., "black disease" in Hindi), with symptoms such as fever, weight loss, anemia and splenomegaly and hepatomegaly, which often ends in death in untreated people.
Despite the huge impact on human health, leishmaniasis belongs to the category of neglected diseases. This is because it mainly affects people in the world's poorest countries. Therefore, it is the duty of the more developed countries to help in the fight against this disease. A thorough understanding of the biology and epidemiology of leishmania is an essential prerequisite for successful control and treatment. This is what we are trying to do in our laboratory, even though human cases of leishmaniasis in our country have so far only been imported.
Q: To help our readers understand who is conducting this important work, can you tell us more about the people in the lab and research teams?
A: The head of the laboratory is Prof. Petr Volf, a scientist who has been studying sand flies and leishmaniases for about thirty years. The lab consists of several groups, with Prof. Volf leading a group focusing on sand fly salivary proteins and the host immune response to insect bites.
Dr. Vit Dvorak and his students are involved in field studies, focusing on molecular taxonomy of sand flies and Leishmania epidemiology. Two groups investigating insect immunity and sand fly-borne viruses are led by Dr. Erich Telleria and Dr. Magdalena Jancarova, respectively.
 I lead the team focusing on experimental infections of sand flies and rodents.
I lead the team focusing on experimental infections of sand flies and rodents.
We study mainly factors controlling vector competence and Leishmania virulence factors, but we are also interested in research of alternative vectors of leishmania, incrimination of reservoir hosts and animal models.
The most important members of my group are our post-doc Barbara Vojtková and PhD student Tomas Becvar.
Q: How are you applying the Harpera device in your current research project?
Our current project, supported by the Czech Science Foundation, is aimed at research on the transmission of Leishmania major, parasite species causing CL in the Old World.
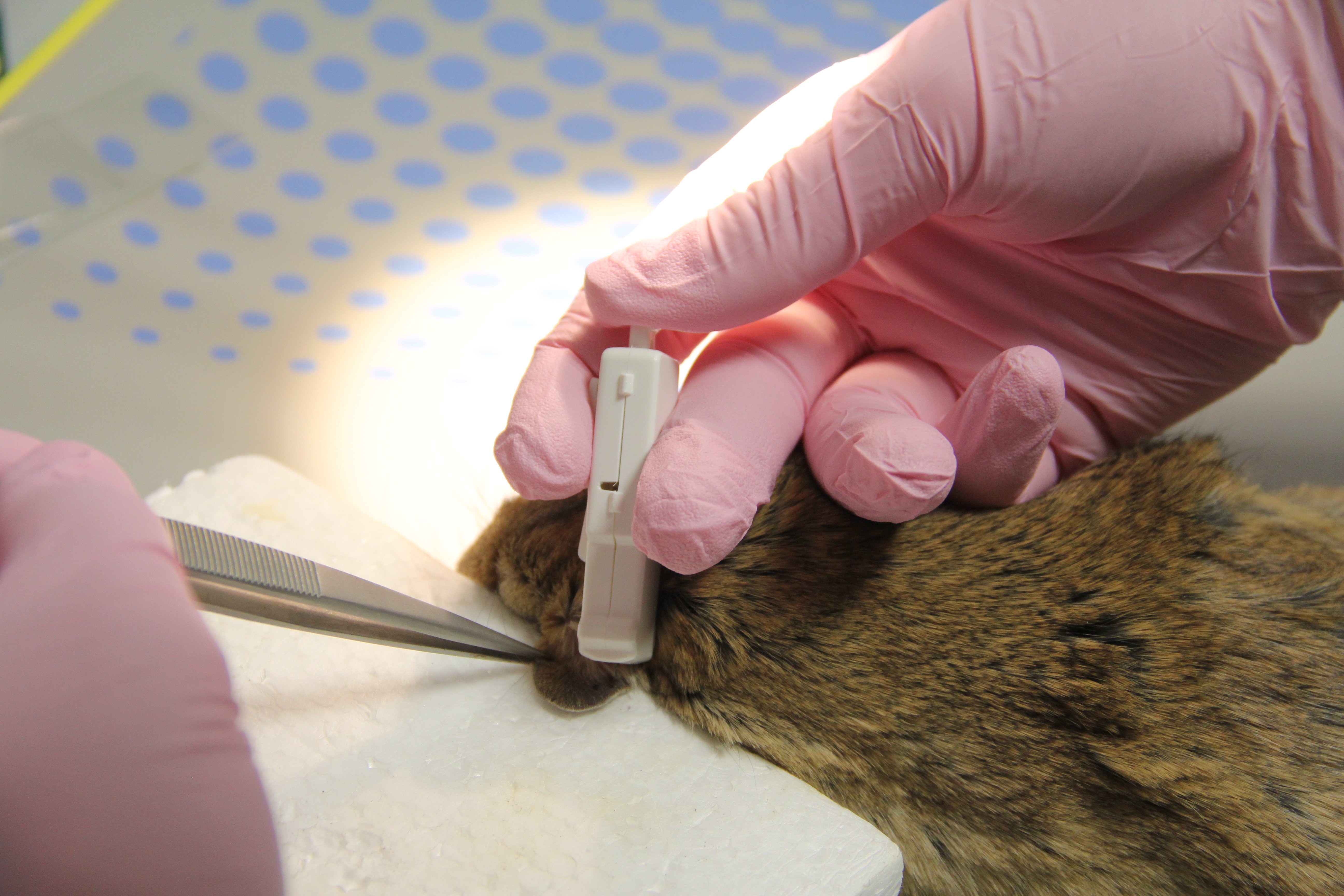 We are using Harpera to collect skin samples from rodents. With our analysis of the skin samples, we aim to reveal important general transmission parameters, crucial for understanding of Leishmania epidemiology.
We are using Harpera to collect skin samples from rodents. With our analysis of the skin samples, we aim to reveal important general transmission parameters, crucial for understanding of Leishmania epidemiology.
Specifically, we want to study Leishmania transmissibility in symptomatic and asymptomatic hosts, spatial distribution of the parasite in the host body and numbers of Leishmania necessary for establishment of sand fly infection.
The project also has a field component aimed at identifying insect vectors and rodent reservoir hosts in Algeria, the country with the second highest annual number of cutaneous leishmaniasis cases worldwide.
Q: Can you provide more information about the rodent species you are sampling as part of the leishmaniasis study?
A: Yes, it was crucial to have a good laboratory model for this project. In collaboration with Algerian colleagues, we therefore established a breeding colony of Meriones shawi, the natural rodent reservoir of L. major in North Africa. We also obtained an isolate of L. major from the same endemic locality in Algeria, and we have available the colony of Phlebotomus papatasi, the proven vector of L. major in this region.
Q: What advantage does the Harpera™ device provide that wasn’t available to you with other tools/methods? (Do you need to collect multiple biopsies from many study animals at different time points? Please explain this and how Harpera helps.)
A: It has recently been described that leishmania are distributed heterogeneously in host tissues, which influences the transmissibility of the parasite to vectors. Previous studies on this topic have been done on a single time point post-infection and used immunodeficient mice as a model.
 We aim to show for the first time the kinetics of the parasite distribution in the natural host.
We aim to show for the first time the kinetics of the parasite distribution in the natural host.
Non-invasive skin sampling using the Harpera device helps us to do just that, because with Microbiopsy we can take such a small piece of tissue that we can examine the site repeatedly and thus observe changes in leishmania localization over time.
Another task of ours is to evaluate the number of skin parasites needed to establish sand fly infection.
 Also in this respect, Harpera is a unique because it mimics the mode by which phlebotomine sand flies acquire blood meals. The Harpera device penetrates the skin to a depth of ~200 µm (similar to the length of a sand fly proboscis) and samples a small number of skin cells, comparable to the volume taken up by a female sand fly.
Also in this respect, Harpera is a unique because it mimics the mode by which phlebotomine sand flies acquire blood meals. The Harpera device penetrates the skin to a depth of ~200 µm (similar to the length of a sand fly proboscis) and samples a small number of skin cells, comparable to the volume taken up by a female sand fly.
Q: What are your experiences so far with using the Harpera? (For example, I believe with a traditional biopsy on a mouse, the biopsy is painful to the animal and after multiple samples are taken, the animal must be euthanized. For additional biopsy, you only collect the final samples after the animal has died, correct? Can you explain how traditional biopsy differs from minimally invasive microbiopsy using Harpera, and what impact this change has on the animals and your results?)
A: Yes, you're right on all points. Conventional biopsy takes an incomparably larger piece of tissue, which is painful and makes it impossible to sample the same spot again. The traditional biopsy method would also not work at all on the microscale we are interested in. Microbiopsies thus combines better welfare for experimental animals while allowing us to explore the details of parasite localization that we could not otherwise detect.
Q: Are you publishing the results for your work soon and, if so, where can our readers learn more about your study?
A: We will soon publish the first part of the research conducted on our M. shawi - L. major model, which focuses on the infectivity of asymptomatic animals. Previously, it was assumed that the vector becomes infected by feeding on a skin lesion where leishmania are accumulated.
We have performed a series of more than 100 xenodiagnostic experiments and observed that animals without obvious external symptoms on the skin are as infectious to the vector as symptomatic hosts and, thus, play a significant role in the transmission of L. major. This article should soon appear in the Special Issue "Host-Pathogen Interactions during Leishmania Infections" of the journal Pathogens.
Q: What are next steps for your research team & what is your ultimate goal with this work?
A: We want to complete research on the distribution of Leishmania in the host skin, while we also continue working on incrimination of the host competence of some North African rodent species for L. major.
We have also started research on the competence of European sand fly species for Leishmania species that are spreading due to climate change and mass population migrations.
Another interesting chapter is the Mundinia subgenus of Leishmania. It is a group of six species described only a few years ago, yet its representatives have been discovered all over the world, including unique areas such as Australia, North America and Central Europe. These parasites are apparently transmitted by biting midges (Ceratopogonidae) unlike the "traditional" leishmania of the other subgenera transmitted by sand flies. Their reservoir hosts are completely unknown.
Summing up, there are a lot of interesting topics and there is no danger of boredom in leishmaniasis research!
We are grateful to Dr. Sádlová and the entire research team at Charles University for sharing details of their work. The Harpera device is currently supplied for Investigational Use Only (IUO).
Image Credits: Jovana Sádlová, Charles University, Trajan
Related Reading:
Kirstein, O.D., et al. (2017). "Minimally invasive microbiopsies: a novel sampling method for identifying asymptomatic, potentially infectious carriers of Leishmania donovani." Int J Parasitol 47(10-11): 609-616.
Churiso,G.,et al. (2020). "Minimally Invasive Microbiopsies as an Improved Sampling Method for the Diagnosis of Cutaneous Leishmaniasis." Open Forum Infect Dis 7(9): ofaa364.
Cloots, K.,et al. (2021). "Assessing L. donovani Skin Parasite Load: A Proof of Concept Study of a Microbiopsy Device in an Indian Setting." Front Cell Infect Microbiol 11: 645121.

Share this
- Microsampling (206)
- Research, Remote Research (119)
- Venipuncture Alternative (105)
- Clinical Trials, Clinical Research (83)
- Mitra® Device (73)
- Therapeutic Drug Monitoring, TDM (51)
- Dried Blood Spot, DBS (39)
- Biomonitoring, Health, Wellness (30)
- Infectious Disease, Vaccines, COVID-19 (24)
- Blood Microsampling, Serology (23)
- Omics, Multi-Omics (21)
- Decentralized Clinical Trial (DCT) (20)
- Specimen Collection (18)
- Toxicology, Doping, Drug/Alcohol Monitoring, PEth (17)
- Skin Microsampling, Microbiopsy (14)
- hemaPEN® Device (13)
- Preclinical Research, Animal Studies (12)
- Pharmaceuticals, Drug Development (9)
- Harpera Device (7)
- Industry News, Microsampling News (5)
- Antibodies, MAbs (3)
- Company Press Release, Product Press Release (3)
- Environmental Toxins, Exposures (1)
- July 2025 (1)
- May 2025 (1)
- April 2025 (2)
- December 2024 (2)
- November 2024 (1)
- October 2024 (3)
- September 2024 (1)
- June 2024 (1)
- May 2024 (1)
- April 2024 (4)
- March 2024 (1)
- February 2024 (2)
- January 2024 (4)
- December 2023 (3)
- November 2023 (3)
- October 2023 (3)
- September 2023 (3)
- July 2023 (3)
- June 2023 (2)
- April 2023 (2)
- March 2023 (2)
- February 2023 (2)
- January 2023 (3)
- December 2022 (2)
- November 2022 (3)
- October 2022 (4)
- September 2022 (3)
- August 2022 (5)
- July 2022 (2)
- June 2022 (2)
- May 2022 (4)
- April 2022 (3)
- March 2022 (3)
- February 2022 (4)
- January 2022 (5)
- December 2021 (3)
- November 2021 (5)
- October 2021 (3)
- September 2021 (3)
- August 2021 (4)
- July 2021 (4)
- June 2021 (4)
- May 2021 (4)
- April 2021 (3)
- March 2021 (5)
- February 2021 (4)
- January 2021 (4)
- December 2020 (3)
- November 2020 (5)
- October 2020 (4)
- September 2020 (3)
- August 2020 (3)
- July 2020 (6)
- June 2020 (4)
- May 2020 (4)
- April 2020 (3)
- March 2020 (6)
- February 2020 (3)
- January 2020 (4)
- December 2019 (5)
- November 2019 (4)
- October 2019 (2)
- September 2019 (4)
- August 2019 (4)
- July 2019 (3)
- June 2019 (7)
- May 2019 (6)
- April 2019 (5)
- March 2019 (6)
- February 2019 (5)
- January 2019 (8)
- December 2018 (3)
- November 2018 (4)
- October 2018 (7)
- September 2018 (6)
- August 2018 (5)
- July 2018 (8)
- June 2018 (6)
- May 2018 (5)
- April 2018 (6)
- March 2018 (4)
- February 2018 (6)
- January 2018 (4)
- December 2017 (2)
- November 2017 (3)
- October 2017 (2)
- September 2017 (4)
- August 2017 (2)
- July 2017 (4)
- June 2017 (5)
- May 2017 (6)
- April 2017 (6)
- March 2017 (5)
- February 2017 (4)
- January 2017 (1)
- July 2016 (3)
- May 2016 (1)
- April 2016 (2)


No Comments Yet
Let us know what you think