Share this
cytokines as predictive biomarkers of severe COVID-19: could at-home sampling be helpful?
by James Rudge, PhD, Technical Director, Trajan on Jul 6, 2020 2:15:00 PM
Since the early 20th Century, many scientists have been interested in investigating cell cultures and the biological properties of molecules secreted by cells, most of which are small protein molecules. In 1974, biochemist Stanley Cohen, PhD, and his colleagues were credited with naming these "cytokines."
What are cytokines?
Cytokines are low molecular weight proteins secreted from white blood cells and a variety of other cells. They get their name from the Greek “cyto” (cells) and “kines” (movement). Their function is to act as molecular messengers that are synthesized as a response to stimuli. They are part of a symphony of intracellular and extracellular molecular interactions that modulate the immune system.
There are four major classes of cytokines. For three of these, the name is derived from the cells that synthesize the specific class of molecular messenger. Monokines, are synthesized from monocytes, Lymphokines from T-cells, and Interleukins from leukocytes. The final class, chemokines, derive their name from their chemotactic function. There are six major families of cytokines including families of Interleukin 1, Hemoprotein, Tumor Necrosis factor, Interleukin 17 and Chemokine F.
Over the years, as scientists attempted to untangle the complexities of the immune system, certain cytokines were “discovered” several times and given a variety of names, until the scientific community realized these new discoveries were, in fact, the same molecule. Indeed, IL-6 has been “discovered” at least four times and was named BSF-2, HSF, HGF and IFN-b2 before finally being renamed as IL-6i.
The reason why cytokines are sometimes “discovered” multiple times, is because they can exert different effects on several cellular types—a behavior known as pleiotropy. In addition to pleiotropic activity, several cytokines can act identically on the same cell type. This is known as redundancy. To complicate matters further, cytokines also can work together synergistically to induce a reaction. Finally, as part of the finetuning of cellular regulation, cytokines can also work antagonistically. For example, IFN-γ acts as an antagonist of IL-4, preventing B cell immunoglobulin class switching.
Like hormones, cytokines are synthesized from a response to stimuli and then interact with cellular receptors to induce an intracellular pathway which may also lead to the production of more cytokines. This process is known as “cascade induction.” The released cytokines may interact with the same cell in an autocrinal fashion. Cytokines may also only interact with cells in the surrounding vicinity, which is known as paracrinal. Furthermore, like hormones, cytokines can show endocrinal activity, affecting cells at a distance being transported by the circulating blood. This makes them interesting biomarker targets.
Cytokines, Inflammation and Viral Infections
Cytokines have several functions: assisting lymphocyte migration, B cell development and gene recombination (to allow adaptation). These molecular messengers also help to mediate other cellular processes, including cell differentiation, T cell activation, cell proliferation and inflammation. It is this latter behavior, inflammation, which has been of great interest to the scientific and medical communities in trying to understand the role that cytokines play in COVID-19 illness, and how this understanding can help inform how we can treat COVID-19 patients.
Observed Cytokine Changes on SARS-COv-2 Infection Since early 2020, scientists and clinicians have been busily investigating the pathology of COVID-19ii. It remains unclear why some people exhibit mild to moderate symptoms, while an estimated 15% go on to develop severe pneumonia, and 5% then progress to acute respiratory distress syndrome, septic shock and/or multi-organ failureiii. It is widely recognized that age and secondary health conditions have a deleterious effect on recovery and correlate with increased morbidity.
Since early 2020, scientists and clinicians have been busily investigating the pathology of COVID-19ii. It remains unclear why some people exhibit mild to moderate symptoms, while an estimated 15% go on to develop severe pneumonia, and 5% then progress to acute respiratory distress syndrome, septic shock and/or multi-organ failureiii. It is widely recognized that age and secondary health conditions have a deleterious effect on recovery and correlate with increased morbidity.
However, there are many examples of younger people with no known secondary health conditions developing severe COVID-19 illness and, sadly, dying from the disease. This has been somewhat puzzling to scientists, but they are now recognizing that a person’s individual immune response plays a major role in final prognosis of COVID-19. It has been reported that people with severe COVID-19 have lymphopenia, showing much reduced levels of CD4+ T cells, CD8+ T cells, B cells, and NK cells, but conversely, an increase in neutrophil countiii.
The same review highlights that people with severe COVID-19 exhibit substantially elevated serum levels of pro-inflammatory cytokines, including IL-6, IL-1B, IL2, IL8, IL-17, G-CSF, GM-CSF, IP10, MCP1, MIP1a and TNF. A recent review on the hematologic, biochemical abnormalities of severe COVID-19 illness and mortality, highlights the fact that IL-6, IL-10 and serum ferritin were strong discriminators of severe COVID-19iv.
A recent publication from Herold et al found that even moderately elevated serum IL-6 levels above 80 pg/mL are sufficient to identify COVID-19 people with a high risk of respiratory failurev. However, this paper noted that it was unclear whether IL-6 merely represented a biomarker or a central pathogenetic factor of severe COVID-19. Nevertheless, Herold et al concluded that IL-6 was an effective marker and may be able to predict imminent respiratory failure, thus helping physicians identify at-risk patients at an early stage.
Extraction of Cytokines from Mitra Blood Microsampling Devices
Using IL-6, and potentially other biomarkers as early predictors of severe COVID-19 illness, raises a hopeful prospect of earlier identification and intervention of high-risk patients. Indeed, there are ongoing clinical trials investigating whether IL-6 receptor antagonist drugs are reducing the incidence of COVID-19 severe respiratory illnessiii. Currently (at the time of writing), governments in many countries are advising that those with mild COVID-19 symptoms self-isolate and apply for a viral RNA test to confirm SARS-CoV-2 infection.
For many, the illness is mild or moderate and doesn’t warrant hospitalization. However, some individuals require hospitalization when symptoms become severe. An at-home test designed to early-identify COVID-19 patients who have elevated IL-6 levels could help reduce the number of SARS-CoV-2 infected people requiring in-person visits with a healthcare professional. Fewer visits to medical facilities reduces the risk of spreading the virus.
In April 2020, the U.S. National Institutes of Health (NIH) announced a serology prevalence study to measure natural immunity to the SARS-CoV-2 virus on a cohort of up to 10,000 subjectsvi. The NIH chose to use Mitra® microsampling devices and at-home Mitra® Blood Collection Kits to allow study participants to remotely collect blood samples to be sent back to the NIH labs for processing. Since the NIH “SeroSurvey” launched, many other institutions and companies have on-boarded the Mitra microsampling technology for similar serology studies. There also has been increasing interest in the feasibility of using remote sampling to measure other biomarkers, including cytokines.
For the last few years Sangui Biovii, an Australian company considered a pioneer in the field of inflammatory biomarkers, have been running studies to correlate protein profiles with disease. Indeed, it has been recognized that chronic inflammation is a predictor of many conditions, such as diabetes, cardiovascular disease, cancer and neurological disorders caused by autoimmune attack. One big risk factor in developing chronic inflammation is lifestyle factors that impact overall health. Obesity, for example, plays a major deleterious role in several health conditions.
To fully understand the effect lifestyle has on chronic inflammation, Sangui Bio has collaborated with Drop Bioviii, an Australian personalized digital health company, to run several studies to provide samples for technique development and look for correlations in risk factors such as obesity, genetic predisposition and, interestingly, protein profiles that include cytokines.
One such study commissioned by Drop Bio in 2019, involved longitudinal sampling of 122 participants provided 536 remotely collected Mitra blood samples. The analyses were broken up into various sub-groups and from one set of 65 individuals, they quantified 27 individual proteins, mostly cytokines and adipokines, per tip using multiplex immunoassays and ELISAs for CRP and Leptin. Table 1 shows a study-wide summary of protein analyte concentrations and population dispersion from Mitra samplesix.
Table 1. Study-wide summary of protein analyte concentrations and dispersion from Mitra samples. All concentrations expressed in pg/mL.
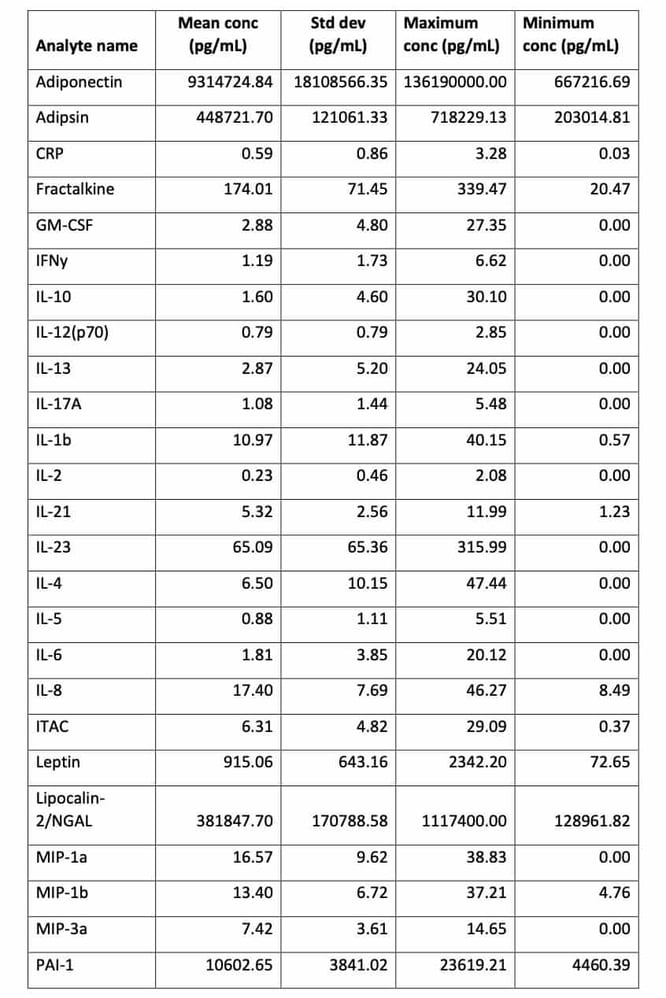
The study demonstrated that cytokines, including low abundance analytes such as IL-6 and IL-1b, can be extracted and quantified from Mitra microsamples self-collected by study participants at home. Given the advantage of remote collection, and the stability of proteins such as antibodies on Mitrax, measurement of predictive biomarkers of severe COVID-19 could be highly useful for early intervention of the disease and longitudinal tracking of COVID survivors. It should be noted that the accuracy and/or precision of cytokine analysis from Mitra tips cannot be assessed from the data presented here.
These data are aggregate results from a large, diverse study population originally designed to illustrate potential dried blood sample reference ranges for a healthy population. They may leave the impression that extraction from Mitra tips introduces variability, however, there is actually a large natural variation of these biomarkers in a human population and cytokines are present in blood cells as well as plasma, which is what these data reflectxi.
On account of the fact that the values from the population study were taken from measuring blood from healthy volunteers, the inflammatory marker ranges are a reflection of that population. This means that, for many, these levels were < 10 pg/mL. Therefore, measuring these markers at such low physiological levels required the use of very sensitive immunoassay equipment, such as the Luminex platform used in this study. Furthermore, the extraction volumes from Mitra (30 uL tips) were lower than is normally recommended (90 uL rather than >300 uL), so care was taken by the investigators to remove as much of the extractant from the tips as possible to recover the necessary volume for detection.
Further details of this work will be found in a follow-up methods application note. Nevertheless, it has to be highlighted that, for IL-6, where >80 pg/mL has shown to be prognostic of further complications with COVID-19, that such extreme measures to pre-concentrate the extract may be unnecessary to develop such a testv. Moreover, in the IL-6 COVID-19 study, the inflammatory biomarker was measured in serum rather than whole blood, so the cut-offs and ranges may well be different. This will also need further investigation.
This article was written by James Rudge, PhD, Microsampling Technical Director, Trajan, and was reviewed by Ben Herbert, PhD, Co-founder and Chief Scientific Officer, Sangui Bio.
References
- i IL-6 in Inflammation, Immunity, and Disease (doi: 10.1101/cshperspect.a016295)
- ii Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China (https://www.thelancet.com/journals/lancet/article/PIIS0140-6736(20)30183-5/fulltext)
- iii COVID-19: immunopathology and its implications for therapy (https://www.nature.com/articles/s41577-020-0308-3.pdf)
- iv Hematologic, biochemical, and immune biomarker abnormalities associated with severe illness and mortality in coronavirus disease 2019 (COVID-19): a meta-analysis (https://doi.org/10.1515/cclm-2020-0369)
- v Level of IL-6 predicts respiratory failure in hospitalized symptomatic COVID-19 patients (https://doi.org/10.1101/2020.04.01.20047381)
- vi https://www.niaid.nih.gov/news-events/nih-begins-study-quantify-undetected-cases-coronavirus-infection
- vii https://www.sanguibio.com/ for more details.
- ix Unpublished data given with consent from DropBio Pty Ltd
- x Application of volumetric absorptive microsampling (VAMS) to measure multidimensional anti-influenza IgG antibodies by the mPlex-Flu assay (https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6885997/)
- xi Karsten, E., Breen, E. & Herbert, B. R. Red blood cells are dynamic reservoirs of cytokines. Scientific Reports 8, article 3101 (2018)
For information on microsampling to research infectious diseases, visit our resources.
Share this
- Microsampling (206)
- Research, Remote Research (119)
- Venipuncture Alternative (105)
- Clinical Trials, Clinical Research (83)
- Mitra® Device (73)
- Therapeutic Drug Monitoring, TDM (51)
- Dried Blood Spot, DBS (39)
- Biomonitoring, Health, Wellness (30)
- Infectious Disease, Vaccines, COVID-19 (24)
- Blood Microsampling, Serology (23)
- Omics, Multi-Omics (21)
- Decentralized Clinical Trial (DCT) (20)
- Specimen Collection (18)
- Toxicology, Doping, Drug/Alcohol Monitoring, PEth (17)
- Skin Microsampling, Microbiopsy (14)
- hemaPEN® Device (13)
- Preclinical Research, Animal Studies (12)
- Pharmaceuticals, Drug Development (9)
- Harpera Device (7)
- Industry News, Microsampling News (5)
- Antibodies, MAbs (3)
- Company Press Release, Product Press Release (3)
- Environmental Toxins, Exposures (1)
- July 2025 (1)
- May 2025 (1)
- April 2025 (2)
- December 2024 (2)
- November 2024 (1)
- October 2024 (3)
- September 2024 (1)
- June 2024 (1)
- May 2024 (1)
- April 2024 (4)
- March 2024 (1)
- February 2024 (2)
- January 2024 (4)
- December 2023 (3)
- November 2023 (3)
- October 2023 (3)
- September 2023 (3)
- July 2023 (3)
- June 2023 (2)
- April 2023 (2)
- March 2023 (2)
- February 2023 (2)
- January 2023 (3)
- December 2022 (2)
- November 2022 (3)
- October 2022 (4)
- September 2022 (3)
- August 2022 (5)
- July 2022 (2)
- June 2022 (2)
- May 2022 (4)
- April 2022 (3)
- March 2022 (3)
- February 2022 (4)
- January 2022 (5)
- December 2021 (3)
- November 2021 (5)
- October 2021 (3)
- September 2021 (3)
- August 2021 (4)
- July 2021 (4)
- June 2021 (4)
- May 2021 (4)
- April 2021 (3)
- March 2021 (5)
- February 2021 (4)
- January 2021 (4)
- December 2020 (3)
- November 2020 (5)
- October 2020 (4)
- September 2020 (3)
- August 2020 (3)
- July 2020 (6)
- June 2020 (4)
- May 2020 (4)
- April 2020 (3)
- March 2020 (6)
- February 2020 (3)
- January 2020 (4)
- December 2019 (5)
- November 2019 (4)
- October 2019 (2)
- September 2019 (4)
- August 2019 (4)
- July 2019 (3)
- June 2019 (7)
- May 2019 (6)
- April 2019 (5)
- March 2019 (6)
- February 2019 (5)
- January 2019 (8)
- December 2018 (3)
- November 2018 (4)
- October 2018 (7)
- September 2018 (6)
- August 2018 (5)
- July 2018 (8)
- June 2018 (6)
- May 2018 (5)
- April 2018 (6)
- March 2018 (4)
- February 2018 (6)
- January 2018 (4)
- December 2017 (2)
- November 2017 (3)
- October 2017 (2)
- September 2017 (4)
- August 2017 (2)
- July 2017 (4)
- June 2017 (5)
- May 2017 (6)
- April 2017 (6)
- March 2017 (5)
- February 2017 (4)
- January 2017 (1)
- July 2016 (3)
- May 2016 (1)
- April 2016 (2)



No Comments Yet
Let us know what you think