Share this
clinical trials: the challenge of subject retention
by Neoteryx Microsampling on Apr 23, 2019 4:20:00 AM
Clinical trials are an important component of research in drug development and in the advancement toward more modern healthcare. After preclinical research has been completed, it is through clinical trials that medications are tested for safety and efficacy.
Without clinical trials that involve human subjects participating in four critical phases to assess the safety and efficacy of new drugs, it would be challenging to make any medical breakthroughs that are formally approved for safe use in the people they are meant to help.

Every potential new medical treatment must go through several clinical trial phases, and each phase has a specific purpose. The different phases are outlined in an example from the MD Anderson Cancer Center, which shows the process followed to assess new oncology medications.
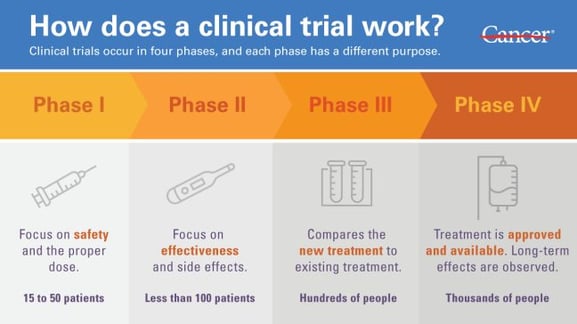
Image Credit: Phases of Clinical Trial, Courtesy of MD Anderson Cancer Center
Like researchers in other scientific fields, medical and pharmaceutical researchers face clinical trial challenges.
Challenge #1: Getting People to Join Clinical Trials as Study Subjects
Recruitment and retention of people for clinical trials can be very challenging. Several factors lead to poor enrollment of study subjects that should be considered in order to run a successful clinical trial, including:
- Types of trials not appropriate for the community being recruited – Diversity in clinical trials is good, but there is often a mismatch between the drug on trial and the types of diseases or conditions that are impacting the targeted community or demographic.
- Insufficient infrastructure support for a community-based clinical trial – Even when trials have an appropriate match between the drug on trial and a community, there can be a lack of sufficient staffing or support to recruit and coordinate study subjects.
- Overly restrictive eligibility requirements – Potential study subjects have reported that they wanted to volunteer for clinical trials but weren't recruited due to strict requirements. There is a need for broader eligibility criteria in trials to include a more diverse patient population, representative of the patients seen in practice.
- Financial barriers – Lack of financial coverage via health insurance or financial incentives for trial participation was reported as a barrier to enrollment in some communities. This barrier is even greater if subjects have to take time away from work and travel long distances to a trial site as part of their participation.
Challenge #2: Retaining People as Study Subjects in Clinical Trials
There are several reasons why subject retention is challenging, including:
- Communication is key: Most clinical trials have a small population of patients who have the conditions being investigated. Once these study subjects have been found, it can be hard to keep them engaged. One reliable means of retaining subjects in these trials is communicating why the research is important. Once the patients are there, it’s important to let them know everything there is to know (that is reasonable to reveal) about the research. A training session in which the patients meet the scientists they will be working can be appropriate. It helps people understand what is expected of them and why they're doing this.
- Fear is a factor: There is often a fear of side effects or fear of painful procedures. This can lead to patients dropping out of the research study (clinical trial) early. Fear usually affects patients at the initial stage of the study just before a trial begins. There is a psychological component that can be eased by creating awareness. Information helps put peoples' minds at ease and lets them know what to expect. Once the trial starts, informed patients are less likely to drop out of the study because of uncertainty; they already know what to expect.
Procedures such as frequent blood draws may cause pain and stress. This could be minimized by using patient-friendly tools that reduce pain and improve the study experience. For example, portable microsampling devices can be used in-clinic or at home to collect blood samples with a quick and easy fingerstick.
Remote microsampling tools like the Mitra® device or hemaPEN® allow for the collection of micro-sized blood samples – just a few drops from a fingertip. These devices also enable remote sample collection with minimal training which, in turn, enables remote monitoring of subjects. They self-collect their samples at home, mail them to the lab for analysis using standard mail, and their clinical trial team follows up via phone, email or video conference. - Changing circumstances: It is difficult to retain patients once a trial has started if the patient’s circumstances change. For example, their family may decide to relocate while the study is underway. An unforeseen distance barrier might make it difficult for a patient to continue participating in the trial, unless there is an option for remote participation that can continue despite a change of location.
Learn how to conduct clinical trials remotely and the benefits of decentralized clinical trials, by visiting our Decentralized Clinical Trials Resource Page:
Image Credits: MD Anderson Cancer Center, Shutterstock, Trajan Scientific and Medical, Neoteryx

Share this
- Microsampling (206)
- Research, Remote Research (119)
- Venipuncture Alternative (105)
- Clinical Trials, Clinical Research (83)
- Mitra® Device (73)
- Therapeutic Drug Monitoring, TDM (51)
- Dried Blood Spot, DBS (39)
- Biomonitoring, Health, Wellness (30)
- Infectious Disease, Vaccines, COVID-19 (24)
- Blood Microsampling, Serology (23)
- Omics, Multi-Omics (21)
- Decentralized Clinical Trial (DCT) (20)
- Specimen Collection (18)
- Toxicology, Doping, Drug/Alcohol Monitoring, PEth (17)
- Skin Microsampling, Microbiopsy (14)
- hemaPEN® Device (13)
- Preclinical Research, Animal Studies (12)
- Pharmaceuticals, Drug Development (9)
- Harpera Device (7)
- Industry News, Microsampling News (5)
- Antibodies, MAbs (3)
- Company Press Release, Product Press Release (3)
- Environmental Toxins, Exposures (1)
- July 2025 (1)
- May 2025 (1)
- April 2025 (2)
- December 2024 (2)
- November 2024 (1)
- October 2024 (3)
- September 2024 (1)
- June 2024 (1)
- May 2024 (1)
- April 2024 (4)
- March 2024 (1)
- February 2024 (2)
- January 2024 (4)
- December 2023 (3)
- November 2023 (3)
- October 2023 (3)
- September 2023 (3)
- July 2023 (3)
- June 2023 (2)
- April 2023 (2)
- March 2023 (2)
- February 2023 (2)
- January 2023 (3)
- December 2022 (2)
- November 2022 (3)
- October 2022 (4)
- September 2022 (3)
- August 2022 (5)
- July 2022 (2)
- June 2022 (2)
- May 2022 (4)
- April 2022 (3)
- March 2022 (3)
- February 2022 (4)
- January 2022 (5)
- December 2021 (3)
- November 2021 (5)
- October 2021 (3)
- September 2021 (3)
- August 2021 (4)
- July 2021 (4)
- June 2021 (4)
- May 2021 (4)
- April 2021 (3)
- March 2021 (5)
- February 2021 (4)
- January 2021 (4)
- December 2020 (3)
- November 2020 (5)
- October 2020 (4)
- September 2020 (3)
- August 2020 (3)
- July 2020 (6)
- June 2020 (4)
- May 2020 (4)
- April 2020 (3)
- March 2020 (6)
- February 2020 (3)
- January 2020 (4)
- December 2019 (5)
- November 2019 (4)
- October 2019 (2)
- September 2019 (4)
- August 2019 (4)
- July 2019 (3)
- June 2019 (7)
- May 2019 (6)
- April 2019 (5)
- March 2019 (6)
- February 2019 (5)
- January 2019 (8)
- December 2018 (3)
- November 2018 (4)
- October 2018 (7)
- September 2018 (6)
- August 2018 (5)
- July 2018 (8)
- June 2018 (6)
- May 2018 (5)
- April 2018 (6)
- March 2018 (4)
- February 2018 (6)
- January 2018 (4)
- December 2017 (2)
- November 2017 (3)
- October 2017 (2)
- September 2017 (4)
- August 2017 (2)
- July 2017 (4)
- June 2017 (5)
- May 2017 (6)
- April 2017 (6)
- March 2017 (5)
- February 2017 (4)
- January 2017 (1)
- July 2016 (3)
- May 2016 (1)
- April 2016 (2)


No Comments Yet
Let us know what you think