Share this
clinical trial: bridging between VAMS and traditional samples
by James Rudge, PhD, Technical Director, Trajan on Feb 28, 2022 9:00:00 AM
This blog begins with a review of an article published by David Sciberras et al at UCB Biopharma and PRA Health sciences in the January 2019 issue of Pharmacology Research and Perspectives. The authors reported on the results of a Phase 1 clinical PK study of a drug under development for the treatment of infantile spasms.
The paper is entitled “A pharmacokinetic study of radiprodil oral suspension in healthy adults comparing conventional venous blood sampling with two microsampling techniques.” It describes a study where PK safety and tolerability of an infant formula was evaluated on healthy adult volunteers.
 For the study, two microsampling approaches (including Mitra® devices with VAMS®) were bridged alongside standard venous draws.
For the study, two microsampling approaches (including Mitra® devices with VAMS®) were bridged alongside standard venous draws.
The study was a success and the investigators reported that the microsampling technique allowed the potential for collection of samples at home or in remote areas where access to a clinic might be challenging. The authors added, “The key limitation of the study's relevance to infants is that it was conducted in adults.”
Details of the Bridging Study
- Study was carried out on 10 healthy volunteers to test an oral suspension for treatment of infant spasms (IS). IS often occur in first year of life, peaking at 3-5 months
- The drug to be tested was Radiprodil (UCB3491), “a selective negative allosteric modulator of NR2B‐containing N‐methyl‐d‐aspartate (NMDA) receptors”
- The primary objective was to conduct a first in human study on healthy adult volunteers to evaluate the pharmacokinetics of the oral suspension. A secondary objective was to test the safety and tolerability in this population. Finally, an exploratory objective was to evaluate two microsampling techniques (Mitra and Aqua‐Cap™ Drummond capillary tubes).
Method Pertaining to Sampling
- The two microsampling techniques (Mitra from Neoteryx and Aqua‐Cap™ from Drummond) that were employed, differed to one another in both volume of collection and final sample matrix type. Mitra collected duplicate samples (10 µL), per time-point and also per venous and also capillary collection.
The samples were dried and whole blood extractions were conducted to measure drug level. The Drummond device collected 70 µL of venous or capillary blood which was then used to harvest plasma (through centrifugation) and test drug level in this wet matrix. - Blood samples were collected at multiple timepoints: “pre-dose, and 0.5, 1, 1.5, 2, 2.5, 3, 3.5, 4, 6, 8, 12, 24, 36, and 48 hours post dose.” Venous blood was collected and prior to centrifugation, both Mitra and Aqua‐Cap were used to sample from the venous blood tubes. Concurrent to venous collection, both microsampling techniques were also used to collect capillary blood from a finger prick.
This allowed for a bridging study to evaluate conventionally venous harvested plasma, venous and capillary plasma harvested from the Aqua‐Cap, and also dried venous & capillary whole blood from Mitra. The authors noted that PK parameters were not calculated for the Aqua‐Cap™ Drummond venous samples, as these were only collected at 2.5, 3, and 3.5 hours.
Study Results, Discussion & Findings
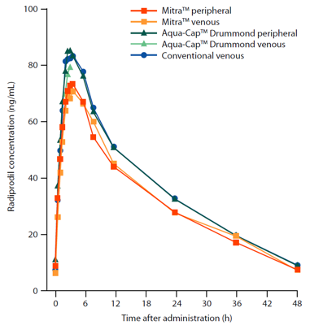
Image: D Sciberras et al, UCB Biopharma & PRA Health sciences, Jan. 2019, Pharmac Res & Perspect.
- An oral suspension of the drug had been developed so that an extemporaneously prepared sample could be made to cover a wide range of doses, allowing for dose adjustment critical for infant settings.
- The suspension had an acceptable safety profile and the taste (10/10) and texture (9/10) were deemed acceptable, which is an important factor in drug compliance for infants and children.
- The study authors reported that “Marginally lower concentrations of radiprodil were observed over the treatment period using peripheral and venous Mitra™ microsampling compared with Aqua‐Cap™ Drummond (peripheral and venous) microsampling and conventional venous sampling.”
- Bioequivalence was demonstrated in terms of the 90% CIs of the geometric mean ratio, regardless of the site of collection of the microsample. It was hypothesized that the difference seen between the whole blood analysis and plasma analysis was as a result of the potential difference between blood to plasma partitioning. The researchers reported that this confirmed that either technique was fit for purpose in future clinical studies with radiprodil.
- The authors stated that Mitra showed benefits over wet sampling, often obviating the need for centrifugation and freezing or cold-chain transport.
- Pre-study bioanalytical method validation of radiprodil demonstrated no HCT bias; which was highlighted by the authors as a concern when considering DBS.
Neoteryx Commentary: The Importance of Bridging
The above review of this excellent 2019 paper demonstrates how critically important bridging is when developing methods involving different matrices. Indeed, the US Food and Drug Administration (FDA) recommended in their 2018 Bioanalytical Method Validation Guidelines (05/24/18) pertaining to blood spots: “Correlative studies with traditional sampling should be conducted during drug development.”
In the case of the UCB bridging study, all matrices independent of location (capillary or venous) and type (plasma or blood), showed statistically equivalent data. However, this may not always be the case. Indeed, even within this study, there was a notable difference in absolute concentration when comparing dried blood vs. plasma. This was attributed to blood-to-plasma partitioning ratios as a root cause.
Indeed, B:P ratio can be very significant and must always be accounted for when validating methods with capillary blood. For example, a published study by David J. Marshall et al (reviewed in a previous blog), showed that when comparing steroid hormone levels in dried whole blood from VAMS extracts to wet plasma, the plasma bound hormones showed a negative bias in the VAMS extracts. This is because these hormones are not usually found in the hematocrit (HCT) in significant quantities.
As a result, to arrive at plasma equivalent values, the group employed a HCT compensation measure (by measuring Hb levels as a surrogate HCT marker) to correct for each datapoint. Indeed, this approach has been used and reported on in many other studies.
There is another consideration when bridging from tractional venous plasma to dried capillary blood: sampling site location (venous vs. capillary). In the UCB study, sampling location was found to not affect the results, but in the Marshall study, one hormone (testosterone) out of the three evaluated showed a significant difference in concentration between both locations.
A final point is that preservatives used in venous blood samples can, on occasion, affect the results. This was recently reported on by Merck Pharmaceuticals, where EDTA was found to affect stable labelled internal standard levels.
Indeed, the need for such a bridge is highlighted in the recently published Official International Association for Therapeutic Drug Monitoring and Clinical Toxicology Guidelines, where it states “It is essential that the use of this anticoagulant does not impact the obtained results, and that the stability of calibrators and QCs reflects that of real samples. Hence, we strongly advise to compare in an early-stage results obtained from a non-anticoagulated sample with results from patient samples anticoagulated with different anticoagulants.”
As volumetric dried blood microsampling continues to gain traction, it is critical that extra care is taken to fully validate studies employing these technologies. When conducted appropriately, as evidenced by the studies highlighted in this technical blog, robust assays can be fully validated, giving researchers a choice as to how to collect high-quality samples from hard-to-reach cohorts.
This study paper was summarized for our readers by James Rudge, PhD, Neoteryx Technical Director. This is curated content. To learn more about the important research outlined in this review, visit the original article published in the journal, Pharmacology Research and Perspectives.

You can access this paper and others on studies using microsampling in our Technical Resource Library.
Share this
- Microsampling (206)
- Research, Remote Research (119)
- Venipuncture Alternative (105)
- Clinical Trials, Clinical Research (83)
- Mitra® Device (73)
- Therapeutic Drug Monitoring, TDM (51)
- Dried Blood Spot, DBS (39)
- Biomonitoring, Health, Wellness (30)
- Infectious Disease, Vaccines, COVID-19 (24)
- Blood Microsampling, Serology (23)
- Omics, Multi-Omics (21)
- Decentralized Clinical Trial (DCT) (20)
- Specimen Collection (18)
- Toxicology, Doping, Drug/Alcohol Monitoring, PEth (17)
- Skin Microsampling, Microbiopsy (14)
- hemaPEN® Device (13)
- Preclinical Research, Animal Studies (12)
- Pharmaceuticals, Drug Development (9)
- Harpera Device (7)
- Industry News, Microsampling News (5)
- Antibodies, MAbs (3)
- Company Press Release, Product Press Release (3)
- Environmental Toxins, Exposures (1)
- July 2025 (1)
- May 2025 (1)
- April 2025 (2)
- December 2024 (2)
- November 2024 (1)
- October 2024 (3)
- September 2024 (1)
- June 2024 (1)
- May 2024 (1)
- April 2024 (4)
- March 2024 (1)
- February 2024 (2)
- January 2024 (4)
- December 2023 (3)
- November 2023 (3)
- October 2023 (3)
- September 2023 (3)
- July 2023 (3)
- June 2023 (2)
- April 2023 (2)
- March 2023 (2)
- February 2023 (2)
- January 2023 (3)
- December 2022 (2)
- November 2022 (3)
- October 2022 (4)
- September 2022 (3)
- August 2022 (5)
- July 2022 (2)
- June 2022 (2)
- May 2022 (4)
- April 2022 (3)
- March 2022 (3)
- February 2022 (4)
- January 2022 (5)
- December 2021 (3)
- November 2021 (5)
- October 2021 (3)
- September 2021 (3)
- August 2021 (4)
- July 2021 (4)
- June 2021 (4)
- May 2021 (4)
- April 2021 (3)
- March 2021 (5)
- February 2021 (4)
- January 2021 (4)
- December 2020 (3)
- November 2020 (5)
- October 2020 (4)
- September 2020 (3)
- August 2020 (3)
- July 2020 (6)
- June 2020 (4)
- May 2020 (4)
- April 2020 (3)
- March 2020 (6)
- February 2020 (3)
- January 2020 (4)
- December 2019 (5)
- November 2019 (4)
- October 2019 (2)
- September 2019 (4)
- August 2019 (4)
- July 2019 (3)
- June 2019 (7)
- May 2019 (6)
- April 2019 (5)
- March 2019 (6)
- February 2019 (5)
- January 2019 (8)
- December 2018 (3)
- November 2018 (4)
- October 2018 (7)
- September 2018 (6)
- August 2018 (5)
- July 2018 (8)
- June 2018 (6)
- May 2018 (5)
- April 2018 (6)
- March 2018 (4)
- February 2018 (6)
- January 2018 (4)
- December 2017 (2)
- November 2017 (3)
- October 2017 (2)
- September 2017 (4)
- August 2017 (2)
- July 2017 (4)
- June 2017 (5)
- May 2017 (6)
- April 2017 (6)
- March 2017 (5)
- February 2017 (4)
- January 2017 (1)
- July 2016 (3)
- May 2016 (1)
- April 2016 (2)


No Comments Yet
Let us know what you think