Share this
Why consider two volumetric blood microsampling devices?
by James Rudge, PhD, Technical Director, Trajan on Jan 30, 2023 9:00:00 AM
When Robert Guthrie started using the dried blood spot (DBS) technique as a diagnostic in 1963 for collecting blood samples from newborns via a quick heel-stick, he created an easy way to screen neonates for congenital disorders, such as inborn errors of metabolism (e.g., phenylketonuria).
The DBS technique using a heel-stick and collecting a blood drop onto filter paper for early diagnosis of disorders transformed newborn screening in hospitals over the ensuing years. A major benefit of the DBS technique was that it allowed for microsamples of capillary blood to be collected without the need for phlebotomy to be performed by specialists trained in accessing the delicate and tiny veins of newborns.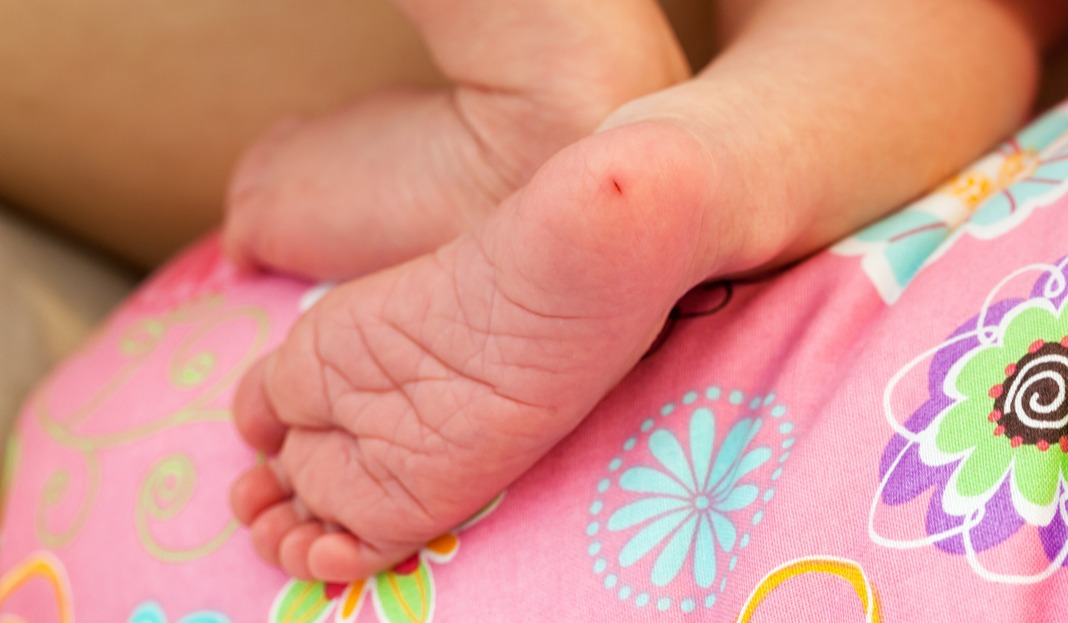
DBS Opened New Avenues for Blood Sampling
Because DBS could be applied by a wider range of professionals without the need for specialized training in venipuncture, it became an important technique for collecting capillary dried blood microsamples for a variety of applications.
These applications currently range from analysis of small drugs to large protein molecules. Furthermore, the DBS technique with a finger-stick is amenable to practically any age group, not just neonates, and has since been applied in many research studies and public health programs involving both adult and pediatric subjects. However, DBS microsampling is not without its shortcomings. The DBS shortcomings are related to both sample quality issues that lead to high sample failure rates and to challenges with quantitation. The latter has been associated with a lack of volume control when blood drops are applied to the filter paper or card. Also, problems with hematocrit occur because of the way blood spreads across the DBS card, which can lead to biases in analytical data.
However, DBS microsampling is not without its shortcomings. The DBS shortcomings are related to both sample quality issues that lead to high sample failure rates and to challenges with quantitation. The latter has been associated with a lack of volume control when blood drops are applied to the filter paper or card. Also, problems with hematocrit occur because of the way blood spreads across the DBS card, which can lead to biases in analytical data.
To address both the quantitation and quality issues, several research groups and companies embarked on projects to search for solutions that might solve the shortcomings observed with DBS. One such solution was to experiment with internal standard (IS) in bioanalytical workflows to improve analytical biases.
At the same time, companies like Trajan Scientific and Medical (Trajan) and Neoteryx began to explore a means to obtain a volumetrically collected microsample.
Launch of the Mitra Device
 In 2014, Neoteryx launched a volumetric absorptive microsampling tool called the Mitra® device. Based on a patented VAMS® technology that is delivered via polymeric tips mounted onto plastic microsampler bodies that look like pipette tips, Mitra devices are designed for remote collection of a fixed volume of capillary blood independent of percent hematocrit. The devices are housed in protective device cartridges (with 2, 3 or 4 microsamplers per pack) and shipped to end-users enclosed in an outer foil specimen bag containing a pouch of drying desiccant.
In 2014, Neoteryx launched a volumetric absorptive microsampling tool called the Mitra® device. Based on a patented VAMS® technology that is delivered via polymeric tips mounted onto plastic microsampler bodies that look like pipette tips, Mitra devices are designed for remote collection of a fixed volume of capillary blood independent of percent hematocrit. The devices are housed in protective device cartridges (with 2, 3 or 4 microsamplers per pack) and shipped to end-users enclosed in an outer foil specimen bag containing a pouch of drying desiccant.
After samples are collected, the sampled Mitra devices can be replaced inside the foil specimen bags and put in a shipping envelope for posting back to a central lab via standard mail. The microsamples will dry in transit to the laboratory where they are processed either manually or using regular laboratory automation.
Initially, Mitra devices were launched in just a 10 µL format, but 20 µL and 30 µL tip formats were later developed to allow for access to more sensitive assays, such as next-generation sequencing and challenging ligand binding assays.
Launch of the hemaPEN Device
/2022%20Mitra,%20hemaPEN%20Product%20Shots%20(approved)/lightbox-hemapen-lancet-bandaid-xtr-brit.jpeg?width=323&height=215&name=lightbox-hemapen-lancet-bandaid-xtr-brit.jpeg) Roughly one year after the launch of the Mitra device, Trajan Scientific and Medical launched the hemaPEN® device. Based on a combination of glass capillaries and DBS technology, the hemaPEN is another radically designed and game-changing volumetric blood microsampling product. The hemaPEN collects 4 x 2.74 µL samples onto either Whatman 903 or Perkin Elmer 226 DBS disks (3.5 mm), which are housed in a cartridge securely located within the sampling device. As with the Mitra device, sampling with the hemaPEN is easy and straightforward.
Roughly one year after the launch of the Mitra device, Trajan Scientific and Medical launched the hemaPEN® device. Based on a combination of glass capillaries and DBS technology, the hemaPEN is another radically designed and game-changing volumetric blood microsampling product. The hemaPEN collects 4 x 2.74 µL samples onto either Whatman 903 or Perkin Elmer 226 DBS disks (3.5 mm), which are housed in a cartridge securely located within the sampling device. As with the Mitra device, sampling with the hemaPEN is easy and straightforward.
From a single finger-stick, the end-user experiences rapid and simultaneous filling of four glass capillaries contained inside the distal end (point) of the pen-shaped device. Unlike using standard glass capillaries, however, the capillaries in the hemaPEN device are protected to prevent breakage, which is a risk when sampling blood with traditional glass capillaries.
Once all four capillaries have filled with the four blood samples, the end-user pushes the hemaPEN back into its base, tip down, until they hear a “click.” This clicking action locks the device to prevent tampering and simultaneously allows the capillaries to transfer the four blood samples into the cartridge containing four DBS disks.
Thanks to integrated desiccant inside the hemaPEN, the four DBS samples will dry during transit to a laboratory. The nature of the hemaPEN sampling procedure is such that it is impossible to under- or over-fill the DBS disks because, once the capillaries are full, they will not overfill.
If the capillaries are only partially filled, they will not transfer blood onto the DBS disks, which means the lab will not receive low-volume, low-quality samples.
Combining Multiple Microsampling Products in One Portfolio

In December 2021, Neoteryx became part of Trajan Scientific and Medical as its microsampling product brand. Trajan’s vision is to enable science that benefits people and its purpose is to enrich personal health through scientific tools and solutions.
Trajan is focused on emerging technologies that offer portability, miniaturization, and affordability for the greatest benefit to society. Blood microsampling products are key to the company’s vision and combining the expertise of both Trajan and Neoteryx has allowed Trajan to become a world leader in patient-centric volumetric capillary microsampling.
Why have two microsampling products?
Although on the face of it both Mitra and hemaPEN look like similar solutions, they each provide the user with distinct advantages in different scenarios to allow for choosing the correct microsampling device for assay development and deployment.
We have written an application note that discusses the distinct advantages of both hemaPEN and Mitra in different cases. Information covered in the soon-to-be-released app note includes:
- An in-depth description of both sampling devices
- A focus on each sampling technique
- How both solutions can be readily adopted by standard bioanalytical laboratories using standard laboratory equipment and consumables
- How method development and validation can be streamlined by using laboratory optimized versions of the patient-centric products
- How to choose the right microsampling solution for your project:
- What sample volume do you need for your assay (2.47 µL – 120 µL)?
- What absorptive material do you require (paper or polymer)?
- Do you need tamper resistance?
- Do you need an integrated drying desiccant?
- Do you need high throughput lab processing?
Thoughts on the Microsampling Landscape
The pandemic showed that large-scale clinical studies could be successfully conducted solely from remote microsamples collected by people in their own homes.
Study participants relied on illustrated instructions included inside remote sampling kits and instructional videos available online to understand how to use microsampling devices to self-collect blood samples.
These remote studies were so successful that the demand for high-precision (<5% RSD), easy to use, patient-centric volumetric microsampling devices has increased substantially.
Feedback from researchers and clinical trial coordinators indicates that minimally invasive and remote blood sampling helps to overcome some barriers to study recruitment, compliance, and retention.
Rather than requiring study participants to endure painful venous collection in a clinical setting, allowing people to remotely collect capillary blood from a quick finger-stick has opened a field of opportunity for accessing much larger study cohorts.
Microsampling, coupled with the huge developments in analytical instrument sensitivity and specificity, have converged to make, next generation assays possible. Trajan realizes that there is not a one-size-fits-all solution and that scientists need a variety of microsampling tools such as Mitra and hemaPEN for distinct and novel applications.
Trajan continues growing its microsampling portfolio with more innovations and developments in the pipeline. Indeed, last year the company licensed a skin biopsy technology that collects skin microbiopsies using a nearly painless lancet-like tool for suture free samples[4]. That new tool has been christened the Harpera™ and is being rolled out for research use only in 2023.
As the field of microsampling continues to evolve, Trajan is developing the tools to turn your next generation assays into practical, deployable solutions. Stay tuned, as this is only the beginning of the Trajan microsampling story.

A new application note, titled "The hemaPEN or Mitra Device: How to choose?" is now available.
Fill out the form below to access the app note and download it as a PDF.

Share this
- Microsampling (206)
- Research, Remote Research (119)
- Venipuncture Alternative (105)
- Clinical Trials, Clinical Research (83)
- Mitra® Device (73)
- Therapeutic Drug Monitoring, TDM (51)
- Dried Blood Spot, DBS (39)
- Biomonitoring, Health, Wellness (30)
- Infectious Disease, Vaccines, COVID-19 (24)
- Blood Microsampling, Serology (23)
- Omics, Multi-Omics (21)
- Decentralized Clinical Trial (DCT) (20)
- Specimen Collection (18)
- Toxicology, Doping, Drug/Alcohol Monitoring, PEth (17)
- Skin Microsampling, Microbiopsy (14)
- hemaPEN® Device (13)
- Preclinical Research, Animal Studies (12)
- Pharmaceuticals, Drug Development (9)
- Harpera Device (7)
- Industry News, Microsampling News (5)
- Antibodies, MAbs (3)
- Company Press Release, Product Press Release (3)
- Environmental Toxins, Exposures (1)
- July 2025 (1)
- May 2025 (1)
- April 2025 (2)
- December 2024 (2)
- November 2024 (1)
- October 2024 (3)
- September 2024 (1)
- June 2024 (1)
- May 2024 (1)
- April 2024 (4)
- March 2024 (1)
- February 2024 (2)
- January 2024 (4)
- December 2023 (3)
- November 2023 (3)
- October 2023 (3)
- September 2023 (3)
- July 2023 (3)
- June 2023 (2)
- April 2023 (2)
- March 2023 (2)
- February 2023 (2)
- January 2023 (3)
- December 2022 (2)
- November 2022 (3)
- October 2022 (4)
- September 2022 (3)
- August 2022 (5)
- July 2022 (2)
- June 2022 (2)
- May 2022 (4)
- April 2022 (3)
- March 2022 (3)
- February 2022 (4)
- January 2022 (5)
- December 2021 (3)
- November 2021 (5)
- October 2021 (3)
- September 2021 (3)
- August 2021 (4)
- July 2021 (4)
- June 2021 (4)
- May 2021 (4)
- April 2021 (3)
- March 2021 (5)
- February 2021 (4)
- January 2021 (4)
- December 2020 (3)
- November 2020 (5)
- October 2020 (4)
- September 2020 (3)
- August 2020 (3)
- July 2020 (6)
- June 2020 (4)
- May 2020 (4)
- April 2020 (3)
- March 2020 (6)
- February 2020 (3)
- January 2020 (4)
- December 2019 (5)
- November 2019 (4)
- October 2019 (2)
- September 2019 (4)
- August 2019 (4)
- July 2019 (3)
- June 2019 (7)
- May 2019 (6)
- April 2019 (5)
- March 2019 (6)
- February 2019 (5)
- January 2019 (8)
- December 2018 (3)
- November 2018 (4)
- October 2018 (7)
- September 2018 (6)
- August 2018 (5)
- July 2018 (8)
- June 2018 (6)
- May 2018 (5)
- April 2018 (6)
- March 2018 (4)
- February 2018 (6)
- January 2018 (4)
- December 2017 (2)
- November 2017 (3)
- October 2017 (2)
- September 2017 (4)
- August 2017 (2)
- July 2017 (4)
- June 2017 (5)
- May 2017 (6)
- April 2017 (6)
- March 2017 (5)
- February 2017 (4)
- January 2017 (1)
- July 2016 (3)
- May 2016 (1)
- April 2016 (2)

Comments (1)