Share this
remote microsampling to monitor children with organ transplants
by James Rudge, PhD, Technical Director, Trajan on Oct 3, 2022 9:00:00 AM
An article by Ingvild Andrea Kindem, MD et al at the University of Oslo, Norway, published in the June 2021 edition of Therapeutic Drug Monitoring, investigated the use of Mitra® devices with VAMS® technology for pre- and post-dose measurements of tacrolimus in pediatrics. The paper is entitled “Tacrolimus Measured in Capillary Volumetric Microsamples in Paediatric Patients – A Cross-Validation Study.” This study measured drug levels from 39 volunteers who used remote Mitra with VAMS sample collection devices at home with a high level of sampling success. The authors concluded that tacrolimus could be measured accurately using VAMS samples for solid organ transplant pediatric patients, and that a home-based therapeutic drug monitoring (TDM) approach was feasible.
Overview of Pediatric Organ Transplants
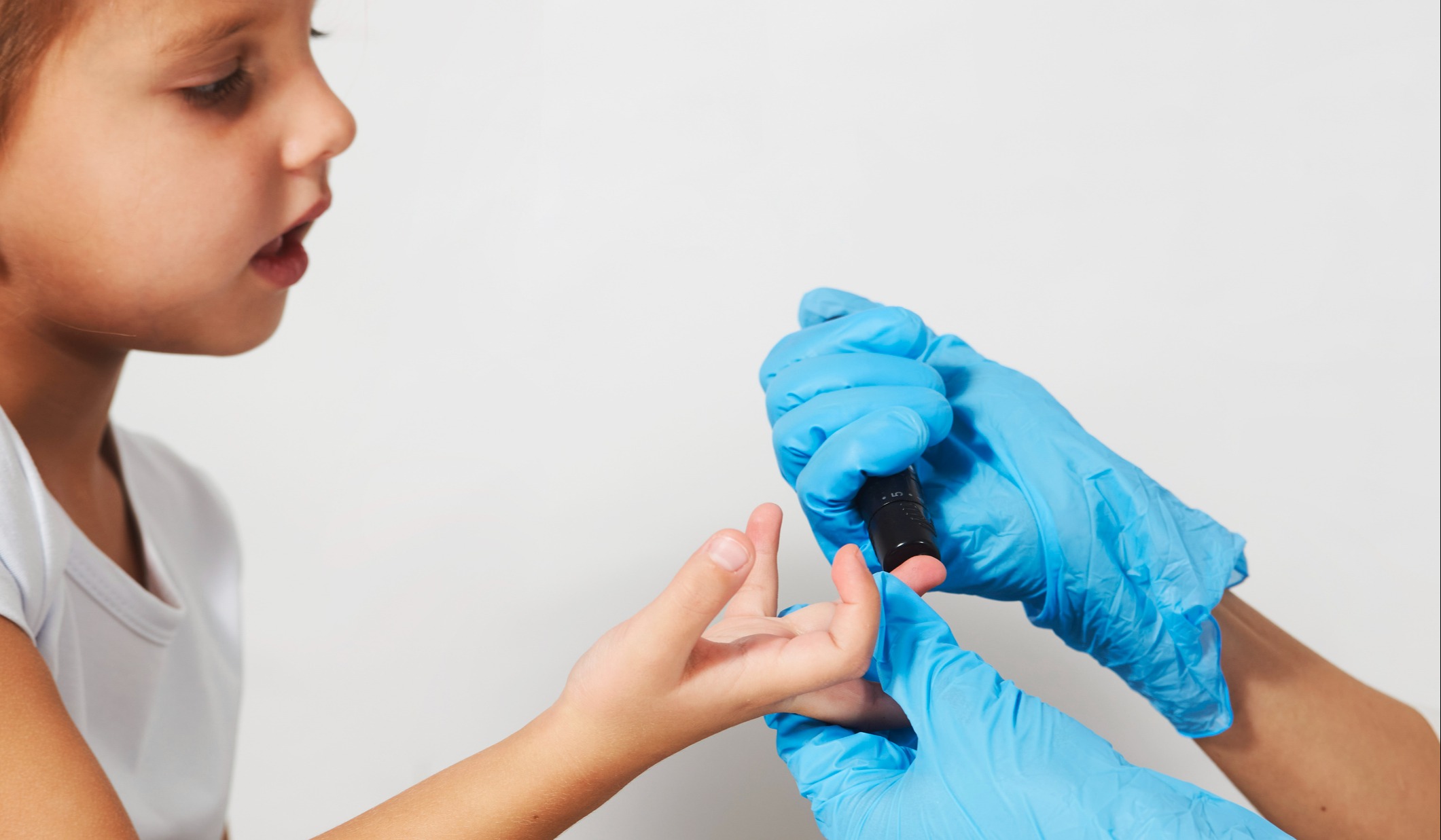 The first pediatric kidney transplant was conducted in 1969 in the United States at the University of Oregon in Portland. Pediatric organ transplant procedures have increased significantly since then, and in the United States and Europe there are now over 1,300 pediatric kidney grafts performed annually, with post-transplant survival rates matching those seen in adults.
The first pediatric kidney transplant was conducted in 1969 in the United States at the University of Oregon in Portland. Pediatric organ transplant procedures have increased significantly since then, and in the United States and Europe there are now over 1,300 pediatric kidney grafts performed annually, with post-transplant survival rates matching those seen in adults.
As in adult cases, the current preferred drug regimen to protect grafted organs in children from graft rejection is to prescribe the calcinuria inhibitor tacrolimus (Tac), with mycophenolate as well as steroids. As discussed in a previous blog, Tac has a narrow therapeutic range where too low a dose leads to rejection and too high a dose leads to complications, such as neurotoxicity, nephrotoxicity post-transplant diabetes, and hypertension. As a result, therapeutic drug monitoring (TDM) for tacrolimus of both adults and children is mandatory to help maintain the health of the grafted organ and the individual. Achieving the right therapeutic balance can be more complicated in children, as growth and development can affect the pharmacokinetics of the drugs, requiring a prolonged and intensive monitoring phase.
Kindem et al, commented that typically 0.5 mL of blood is collected from children either through venipuncture or capillary sampling to measure trough concentrations of Tac. They noted that venipuncture blood draws were not only painful for children, but also stressful. For this reason, the group decided to validate their previously validated VAMS assay that had utilized Mitra devices and a finger-prick method to collect capillary blood from adults for measuring Tac levels in children, too. They hoped that at-home sampling using Mitra devices would also minimize time spent in clinics for the children, would be less burdensome for families, and more cost effective for healthcare providers.
Benefits of Monitoring Children Remotely
Indeed, with only 10 µL needed for reliable measurement of tacrolimus, microsamples could be collected by study volunteers using remote devices in safe and familiar environments such as their homes. The study authors reported that, for some patients, taking samples at home offered an easier and “truer” trough time as compared to the challenge of timing dosing to coincide with clinic visits. Further, their experience with pediatric cohorts indicated that capillary microsampling via a quick finger-stick with a small lancet is more tolerated by children reluctant to have venipuncture with a needle in the arm.
Due to the importance of the accurate determination of Tac concentrations, it was necessary to validate VAMS in pediatric patients prior to deployment. The research group therefore conducted a validation to see if there were any differences between children and adults using both pre-dose trough and post-dose samples.
TDM Study Methods and Findings
- Thirty-nine kidney and liver transplant patients were recruited aged 4-18; the median time for engraftment was 5.1 years.
- Patients (n=38) were sampled at pre-dose for obtaining trough levels.
- Patients were sampled again after 2 hours and given the option to sample again after 5 hours; 4 patients elected to be sampled at 2 hours, and 13 patients elected to be sampled at 5 hours. Three patients elected to be sampled at all 3 timepoints.
- The main reason reported for the lower number of post-dose samples was the patients’ aversion to further venipuncture blood draws.
- A strength of the study was that patients were representative of those who attended the Oslo Clinic.
- The Clinic employed phlebotomists on-call for that day; around 10-12 phlebotomists trained in capillary collection were employed for sample collection.
- Sampling involved simultaneously collecting both venipuncture blood samples and capillary samples (using 2 Mitra devices with VAMS per collection event).
- Order of sampling between capillary collection and venipuncture was randomized.
- Quality of the VAMS samples were high and only one of the 56 samples was rejected due to erroneous filling.
- Two samples were not stored correctly in the specimen bag but were included in the study.
- Analysis was conducted using a previously described LC-MS/MS method for both venipuncture and VAMS samples.
- The results showed that the cross-validation study fulfilled the European Medicines Agency Guidelines on bioanalytical method validation.
- Approximately 90% of the paired samples were within ±20% of the TAC concentrations. Only one sample was >30% and this was a sample that was at a very low concentration (below the therapeutic range). A sample pair with the 2nd highest deviation @ +27% was at a higher drug concentration and no reason for the deviation was identified.
- Passing-Bablock regression analysis saw no bias, either systemic or concentration based.
- When comparing Tac levels to Hemoglobin (Hb, 8.7-15.6 g/dL), no significant correlation was observed. It was concluded that Hb did not affect the results in the range measured.
- Both pre-dose and post-dose samples correlated with standard venous wet samples, which gives the potential for area under the curve (AUC) measurements, something that the group had investigated in a previous study using Mitra devices.
- Sampling at home offers the opportunity for repeated measurements, allowing for closer follow-ups, especially with non-adherent patients.
- Good concordance with VAMS samples (10 µL) was observed compared to standard venous samples.
Neoteryx Comments
It was evident during the study how reticent pediatric participants were to engage in more venipuncture blood draws than was necessary, as only 36% agreed to post-dose sample collection. In contrast, capillary microsampling with a quick finger-stick offers a less stressful and nearly painless sampling experience that would benefit this patient population. Additionally, as the study authors said, finger-stick sampling allows for the possibility of more frequent sampling, which helps to increase surveillance with non-adherent patients. The investigators also observed that this allows for AUC measurements to be taken at home, minimizing extended hospital stays.
This article was summarized for our readers by James Rudge, PhD, Neoteryx Technical Director. This is curated content. To learn more about the important research outlined in this blog, visit the original article in Therapeutic Drug Monitoring.
Image Credits: iStock, Neoteryx, Trajan
For more information on how Neoteryx microsampling products from Trajan benefit researchers, visit our Technical Resource Library.
Share this
- Microsampling (206)
- Research, Remote Research (119)
- Venipuncture Alternative (105)
- Clinical Trials, Clinical Research (83)
- Mitra® Device (73)
- Therapeutic Drug Monitoring, TDM (51)
- Dried Blood Spot, DBS (39)
- Biomonitoring, Health, Wellness (30)
- Infectious Disease, Vaccines, COVID-19 (24)
- Blood Microsampling, Serology (23)
- Omics, Multi-Omics (21)
- Decentralized Clinical Trial (DCT) (20)
- Specimen Collection (18)
- Toxicology, Doping, Drug/Alcohol Monitoring, PEth (17)
- Skin Microsampling, Microbiopsy (14)
- hemaPEN® Device (13)
- Preclinical Research, Animal Studies (12)
- Pharmaceuticals, Drug Development (9)
- Harpera Device (7)
- Industry News, Microsampling News (5)
- Antibodies, MAbs (3)
- Company Press Release, Product Press Release (3)
- Environmental Toxins, Exposures (1)
- July 2025 (1)
- May 2025 (1)
- April 2025 (2)
- December 2024 (2)
- November 2024 (1)
- October 2024 (3)
- September 2024 (1)
- June 2024 (1)
- May 2024 (1)
- April 2024 (4)
- March 2024 (1)
- February 2024 (2)
- January 2024 (4)
- December 2023 (3)
- November 2023 (3)
- October 2023 (3)
- September 2023 (3)
- July 2023 (3)
- June 2023 (2)
- April 2023 (2)
- March 2023 (2)
- February 2023 (2)
- January 2023 (3)
- December 2022 (2)
- November 2022 (3)
- October 2022 (4)
- September 2022 (3)
- August 2022 (5)
- July 2022 (2)
- June 2022 (2)
- May 2022 (4)
- April 2022 (3)
- March 2022 (3)
- February 2022 (4)
- January 2022 (5)
- December 2021 (3)
- November 2021 (5)
- October 2021 (3)
- September 2021 (3)
- August 2021 (4)
- July 2021 (4)
- June 2021 (4)
- May 2021 (4)
- April 2021 (3)
- March 2021 (5)
- February 2021 (4)
- January 2021 (4)
- December 2020 (3)
- November 2020 (5)
- October 2020 (4)
- September 2020 (3)
- August 2020 (3)
- July 2020 (6)
- June 2020 (4)
- May 2020 (4)
- April 2020 (3)
- March 2020 (6)
- February 2020 (3)
- January 2020 (4)
- December 2019 (5)
- November 2019 (4)
- October 2019 (2)
- September 2019 (4)
- August 2019 (4)
- July 2019 (3)
- June 2019 (7)
- May 2019 (6)
- April 2019 (5)
- March 2019 (6)
- February 2019 (5)
- January 2019 (8)
- December 2018 (3)
- November 2018 (4)
- October 2018 (7)
- September 2018 (6)
- August 2018 (5)
- July 2018 (8)
- June 2018 (6)
- May 2018 (5)
- April 2018 (6)
- March 2018 (4)
- February 2018 (6)
- January 2018 (4)
- December 2017 (2)
- November 2017 (3)
- October 2017 (2)
- September 2017 (4)
- August 2017 (2)
- July 2017 (4)
- June 2017 (5)
- May 2017 (6)
- April 2017 (6)
- March 2017 (5)
- February 2017 (4)
- January 2017 (1)
- July 2016 (3)
- May 2016 (1)
- April 2016 (2)



No Comments Yet
Let us know what you think