the viability of Mitra devices for therapeutic drug monitoring
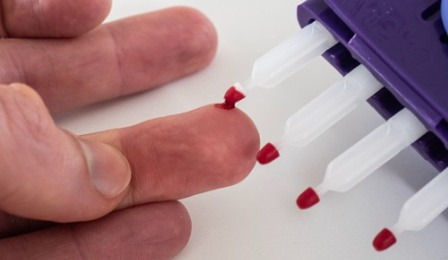

the viability of Mitra devices for therapeutic drug monitoring
Dec 16, 2024 1:32:11 PM
5
min read
remote dried blood sampling offers a solution to metabolite stabilization
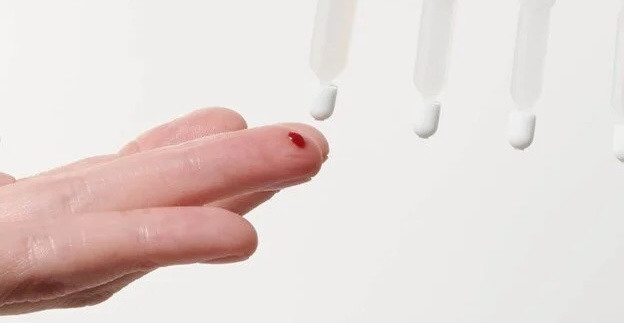

remote dried blood sampling offers a solution to metabolite stabilization
Dec 13, 2024 10:38:38 AM
5
min read
COVID-19 & workplace safety: vaccines & antibodies


COVID-19 & workplace safety: vaccines & antibodies
May 28, 2021 9:00:00 AM
6
min read
why are antibodies critical in our battle against COVID-19?


why are antibodies critical in our battle against COVID-19?
Jun 26, 2020 2:00:00 PM
7
min read
