easy, economical, decentralized specimen collection
flexible microsampling formats for your application
explore VAMS as an alternative to venipuncture
enhance lab efficiency with automated microsampling workflows
microsampling designed with the lab in mind
the gold-standard in remote specimen collection
Powered by VAMS® technology, the patented Mitra® Microsampling Device offers a streamlined approach to biological specimen collection, transport, and preparation. Use Mitra to facilitate sampling direct from animals, humans, or tubes to collect precise, reliable dried microsamples compatible with a wide variety of downstream applications as evidenced by the hundreds of published peer-reviewed manuscripts and the millions of devices that have been used in the field.
Mitra: pioneering VAMS technology
Trajan’s proprietary VAMS® Technology is at the heart of the Mitra device, featuring a sampler tip crafted from a porous, hydrophilic polymeric material. This 'precision sponge' rapidly and consistently absorbs a specific volume of biological fluid—simply by contacting the source. The fluid is then allowed to dry acting as a stabilizer for most analytes.
Available in three tip sizes—10, 20, and 30 µL— and multiple formats, Mitra devices meet diverse end-user and analytical needs. Configurations include dual (2) or quad (4) samplers across various formats. Collect from 20 µL to 120 µL per device based on format selected. Our team of expert microsampling consultants is on hand to provide scientific support, helping you choose the optimal format and workflow for your specific applications.
Reliable
Quantitative sampling with pipette-like precision (RSD ≤ 5%). VAMS eliminates traditional blood hematocrit biases and results in extremely low in-lab sample rejection rates. No glue used in assembly, no matrix interferences.
Versatile
Collect biological fluids—blood, plasma, urine, saliva, and even tears—efficiently from humans and animals (e.g., finger, arm, knee, tail, etc.) or containers. Manual to fully automated workflows are available.
User-Friendly
99% sample acceptance rate validated by studies. VAMS tip acts as a fill indicator to confirm successful sampling. Outer housing protects against contamination and facilitates easy remote or home sampling.
Cost-Effective
Eliminate phlebotomy, couriers, and cold-chain shipping / storage requirements. Devices are compatible with standard automated liquid handling systems and available in various formats to suit different budgets.
successful uses
Have you seen the published, 3rd party literature of how others have successfully implemented Mitra devices?
specimen bag
 The Specimen Bag Mitra format is perfect for those looking to create their own collection kits for remote sampling or maintain bulk devices for centralized sampling in clinical or field settings.
The Specimen Bag Mitra format is perfect for those looking to create their own collection kits for remote sampling or maintain bulk devices for centralized sampling in clinical or field settings.
Each Specimen Bag is sealed and includes a barcoded Mitra device with a unique identifier and desiccant. After collection, the device is placed back into the Specimen Bag to facilitate quick drying and protect the sample from external contamination.
If you need logistics help, bioanalytical lab services, or kitting support, we can connect you with experienced organizations that specialize in microsampling.
Indication for Use: A single-use, non-sterile device used as a specimen collector, and for the storage and transport of blood and other biological fluids for analytical and diagnostic analyses.
Sampler Tip Volume: 10, 20, or 30uL
# of Samplers per Device: 2 or 4
Shelf Life: 18 months
# Device per pack: 30/Pk
Pack content: Mitra Device, Specimen Bag with Desiccant, Sample ID Barcode, 1x IFU
Regulatory status: Mitra® devices are CE-IVD (IVDR) devices intended as a specimen collector, and for the storage and transport of blood and other biological fluids for analytical and diagnostic analyses. They are available as registered IVD Devices in the European Union and United Kingdom, Australia, Brazil, China, and Canada, as well as multiple Health Ministries worldwide. In the USA, Mitra devices are supplied as a research use only (RUO) product to assist in method development, other research-related and non-diagnostic activities. End-users and laboratories must validate the use of the Mitra devices for the particular diagnostic testing intended.
microsample collection kit
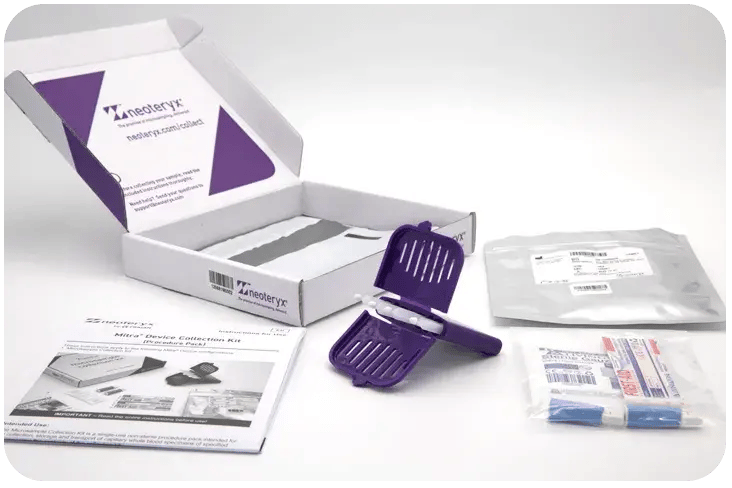
The Microsample Collection Kit Mitra format streamlines the blood microsampling process, enabling both assisted and self-collection at home or in the field. Each kit is equipped with device, barcoding for secure chain of custody, easy-to-follow instructions, all necessary finger-prick sampling supplies, and a return-mailing envelope, all packaged for global distribution. Ideal for remote blood collection, these kits ensure that samples can be directly sent to accredited labs for precise analysis.
For customization to meet the demands of large-scale projects or to optimize your workflows and logistics, please consult with our Microsampling Consultants. They will provide tailored recommendations to align with your project’s specific objectives.
Indication for Use: The Microsample Collection Kit is a single-use non-sterile procedure pack intended for the collection, storage and transport of capillary whole blood specimens of specified volume.
Sampler Tip Volume: 10, 20, or 30uL
# of Samplers per Device: 2 or 4
# Device per kit: 1/Pk
Pack content: 1x Mitra Device, 1x Specimen Bag with Desiccant, Sample ID Barcode, Lancet (x2 or x4), Gauze (x1 or x2), Bandage (x2 or x4), 1x Plastic Shipping Envelope, 1x IFU
Shelf Life: 18 months
Regulatory status: The Mitra Blood Sample Collection Kit is a procedure pack. The kits are available as registered Medical Device in the European Union and United Kingdom, Australia, Brazil, China, and Canada, as well as multiple Health Ministries worldwide. In the USA, procedure packs are supplied as a research use only (RUO) product to assist in method development, other research-related and non-diagnostic activities. End-users and laboratories must validate the use of the Mitra devices and Mitra Microsample collection kit for the particular diagnostic testing intended.
Our Instructions For Use are available in English, German, Danish, Spanish, French, Italian, Dutch, Norwegian, Polish, Japanese, Vietnamese and Portuguese. Need a different language? No problem—just reach out to us at neo.support@trajanscimed.com, and we’ll be happy to assist!
How to Collect a Microsample With VAMS Technology | Step-by-Step Video
English
Française
Italiano
Deutsch
Português
Norsk
96-autorack
 The 96-Autorack™ format is designed for laboratories to efficiently batch and process Mitra microsamples using a standard 96-well format. Compatible with most 1mL & 2mL collection plates and 96-channel pipettors & liquid handling workstations, the 96-Autorack enhances lab productivity and integration.
The 96-Autorack™ format is designed for laboratories to efficiently batch and process Mitra microsamples using a standard 96-well format. Compatible with most 1mL & 2mL collection plates and 96-channel pipettors & liquid handling workstations, the 96-Autorack enhances lab productivity and integration.
We provide two options: an empty 96-Autorack, perfect for accessioning microsamples from Mitra devices received from the field for building plates, extractions, and long-term storage; and prefilled 96-Autoracks with 96 Mitra samplers, ideal for method development and handling calibrators and QCs.
Indication for Use: A single-use, non-sterile device used as a specimen collector, and for the storage and transport of blood and other biological fluids for analytical and diagnostic analyses.
Sampler Tip Volume: 10, 20, or 30uL
# of Samplers per Device: 0 or 96
# Device per pack: 1/Pk
Pack content: 1x 96-Autorack (empty or pre-filled), Sample ID Barcode, 1x Collection Plate, 1x IFU
Shelf Life: 18 months
Regulatory Status: Mitra® devices are CE-IVD (IVDR) devices intended as a specimen collector, and for the storage and transport of blood and other biological fluids for analytical and diagnostic analyses. They are available as registered IVD Devices in the European Union and United Kingdom, Australia, Brazil, China, and Canada, as well as multiple Health Ministries worldwide. In the USA, Mitra devices are supplied as a research use only (RUO) product to assist in method development, other research-related and non-diagnostic activities. End-users and laboratories must validate the use of the Mitra devices for the particular diagnostic testing intended.
storage solution
 The Mitra Device Storage Solution is designed for laboratories to efficiently store for long term sampled Mitra devices in a deep freezer. Compatible to fit one 96-autorack or several Mitra devices, the Mitra Device Storage Solution contain desiccant to keep your samples dry, while the zip closure prevents moisture from entering the bag. A label on the outside of the bag enables samples to be easily marked and identified
The Mitra Device Storage Solution is designed for laboratories to efficiently store for long term sampled Mitra devices in a deep freezer. Compatible to fit one 96-autorack or several Mitra devices, the Mitra Device Storage Solution contain desiccant to keep your samples dry, while the zip closure prevents moisture from entering the bag. A label on the outside of the bag enables samples to be easily marked and identified
Description: Mitra Device Storage Solution
Content: 1x 8”x10” Storage foil bag with 100g (20x 5g) of desiccant, with or without 1x 96-well plate, does not include 96-autorack
Key Features:
- Include desiccants to keep samples dry
- Zip closure to prevent moisture ingress
- Fit all Mitra devices format
- For short and long-term storage of dried Mitra specimens
Stabilize RNA on Your Mitra Microsamples for up to 7 Days!

join the microsampling movement embraced by top organizations around the world.









request pricing information

explore our full range of microsampling products.
/Harpera%202024_IM_4189_15cm_300dpi_RGB-1.jpeg?width=600&height=435&name=Harpera%202024_IM_4189_15cm_300dpi_RGB-1.jpeg)
Harpera Microbiopsy punch
Visit Page
/hemaPEN%20images%20(approved)/IM_3238_RevB_RGB_72dpi_1024px-1.jpeg?width=1024&height=597&name=IM_3238_RevB_RGB_72dpi_1024px-1.jpeg)
hemaPEN DBS Next Generation
Visit Page

GenTegraRNA-NEO Solution
Visit Page
CHRONECT Accessioning and Processing
Visit Page
