welcome to modern, convenient, & precise specimen collection
Advance RNA research with the GenTegraRNA-NEO Active Chemical Protection™ compound, developed for Mitra® microsampling devices.
Accelerate metabolomics with the Metabolon Global Discovery Panel, validated on Mitra® blood microsamples for efficient bioanalysis.
We offer a Microsampling Resource Library of published articles & resources on microsampling studies. Peruse 400+ peer-reviewed resources.
collect dependable microsamples in the lab, at home, in the field or facility
Eliminate the challenges of traditional specimen collection with remote devices based on advanced microsampling technologies that deliver convenience with high precision for reliable data & insights.

Easily collect up to 120 µL of blood and transport by mail.

This volumetric sampling device overcomes many DBS issues.

HarperaTM MicrobiopsyTM
Punch (IUO)
A suture-free, and virtually painless option for scarless skin biopsies.

Apply to Mitra® to stabilize and preserve RNA
join the microsampling movement embraced by top organizations and scientific innovators around the world













… sample collection acceptance rates based on remote specimen collection by end-users

… published materials that demonstrate the scientific accuracy and validity of microsampling.
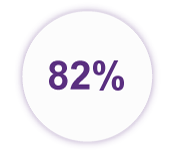
… of people surveyed prefer nearly painless finger-stick sampling vs. stressful needle draws.

… countries where research organizations are publishing on their microsampling studies.
oceans of data from just a few drops
Stay updated with the latest in microsampling technology—where scientific precision meets practical simplicity. Explore news, events, and breakthroughs across various industries.

Mitra® VAMS® Microsampling Collection Video Now Available in Spanish and Arabic

Success with Microsampling in Clinical Trials Live Discussion
Trajan Scientific and Medical, in collaboration with IQVIA Laboratories, announces…

Trajan appoints AppMed Inc. as Canadian distributor for Microsampling technologies, featuring Mitra® and Harpera®
Ringwood, Australia — 4 December 2025 — Trajan Scientific and Medical is pleased t…
microsampling has endless applications
You can apply our personalized sampling solutions to advance research and healthcare.
Leverage microsampling for animal studies & the 3Rs of animal research.
Offer remote sampling options to improve trial success rates.
Collect samples easily from diverse cohorts, including adult & pediatric subjects.
Facilitate remote monitoring of study subjects with at-home sample collection.
Apply microsampling in forensics, PEth monitoring of alcohol & anti-doping.
Use remote collection for disease, serology & epidemiology studies.
Combine glass capillaries + DBS for precision that overcomes hematocrit issues in blood sampling.
Enable biological wellness with samples that reveal environmental exposure & other factors.
Analyze omic data from a precise volume microsample of bio-fluid.
explore, connect, and stay informed

Tech Resource Library
Articles, Bridging Studies & More

Harpera™ Microbiopsy™ Punch
Suitable for a range of clinical and research dermatology applications

Microsampling Ready
Find a laboratory to help you transition to microsampling
innovate your approach to
science and research
Advancing to microsampling begins with a conversation. Contact us!
1 (310) 787-8747 | info@neoteryx.com

