scientific specimen collection, simplified.
our vision & purpose
As the microsampling product brand of Trajan Scientific and Medical, we bring you simple, scientifically precise remote specimen collection devices and microsampling technologies. We are aligned with Trajan’s vision to enrich personal health through scientific tools and solutions. Our microsampling products enable science that benefits people.
our mission
To innovate and develop quantitative microsampling solutions that are easy to use and amenable to manual and automated analysis in the lab. We simplify sample collection and processing for our scientific and medical customers in pharmaceutical development, clinical research, personalized medicine and related industries, enabling them to make advances that positively impact human health.
our technologies
Our microsampling tools and devices facilitate remote specimen collection by anyone, anywhere, anytime. Our products are designed for ease-of-use, convenience, and simplified logistics for sample transport at reduced costs. Our product range enables decentralized research and virtual healthcare models, with a portfolio that includes the Mitra® device based on VAMS® technology, the hemaPEN® based on volumetric DBS technology, and the Harpera ™ MicrobiopsyTM Punch.
the promise of microsampling, delivered.
"We use the blood collection kit at home for our son, who is a kidney transplant recipient. The kit has a Mitra® device, easy instructions, a finger-prick tool & supplies to collect the samples. We use the included shipping envelopes to post samples to our son’s care provider. The kit saves us time and unnecessary trips to the clinic.”
– Mrs. Lego
Transplant Patient Parent, UK
“One of the greatest advantages we have been able to appreciate about Mitra® with VAMS® technology is the simplicity and rapidity of use, while maintaining high performance and guaranteeing the reliability of quali-quantitative analytical data."
– Laura Mercolini, PhD,
Pharmacy and Biotechnology Research Head, University of Bologna, Italy
"VAMS® technology allows convenient at-home monitoring and is minimally invasive. It also offers efficiencies in the clinical setting, as providers will have blood results in hand before meeting with the patient."
– Dr. Christophe Stove
Ghent University Hospital, Belgium
“For our biomarker analysis by chromatography and mass spectrometry using biological samples, the Mitra® with VAMS® technology has allowed us a rapid and effective tuning and validation process, providing a boost to all the optimization steps. In this way, we were able to concentrate on the stability study with a solid and reliable methodology available."
– Michele Protti, Phd
Pharmacy & Biotechnology Research Scientist, University of Bologna, Italy
“Researchers have considerable experience using these at-home blood collection kits to track the spread of other infectious diseases like influenza, and this method is safe, effective and easy-to-use. With a small finger-pick, volunteers can help scientists fight COVID-19 from their homes.”
– Kaitlyn Sadtler, PhD
Study Lead for Laboratory Testing and Chief, NIH NIBIB’s Section for Immunoengineering, US
“One of the greatest advantages we have been able to appreciate about Mitra® with VAMS® technology is the simplicity and rapidity of use, while maintaining high performance and guaranteeing the reliability of quali-quantitative analytical data."
– Laura Mercolini, PhD
Pharmacy and Biotechnology Research Head, University of Bologna, Italy
"Mitra® devices offered ease of sample collection and reduction in the need for trained phlebotomists. The ability to collect samples remotely significantly decreased our logistical burden and costs associated with traditional blood sampling methods. Additionally, the compact size and stability of the samples facilitated easier storage and transportation.”
– Prerna D., PhD,
Researcher, Pharmaceutical Studies, US
our story
Follow our timeline to learn how we developed and grew to where we are today as global leaders in remote specimen collection and microsampling.
A conversation sets VAMS in motion.
Drs. James Rudge and Neil Spooner discuss the limitations of Dried Blood Spot (DBS) cards. This gets James thinking about a dried matrix microsampling tool that will reliably collect fixed-volume samples.

Volumetric absorptive microsampling is born.
Dr. Stuart Kushon and the R&D team at Phenomenex translate James’s idea & prototype for VAMS into a new medical device. Five pharmaceutical companies validate the device, which consistently collects a precise volume of bio-fluid.

Neoteryx LLC is founded in the US.
Neoteryx is formed as a device company with the goal of commercializing tools based on volumetric absorptive microsampling technology. The company offers Mitra® devices with VAMS® in several formats, including the Mitra 96-Autorack for automated, high-throughput processing of multiple samples.

Neoteryx launches VAMS technology.
Mitra® devices based on the original, patented VAMS® technology are registered, trademarked and launched under the Neoteryx banner. Research, healthcare and lab professionals start using Mitra devices with VAMS for a range of applications.
Top organizations adopt Mitra with VAMS.
The NIH, Charles River, and other top science & medicine centers apply Mitra® devices with VAMS® microsampling in pharmaceutical drug discovery & development, clinical research & trials, academic research studies, therapeutic drug monitoring, and public health programs. Hundreds of published studies discuss how VAMS microsampling can be automated for easy workflows and deliver high-quality samples with a 96.8% accuracy rate.

A pandemic spurs wider use of microsampling.
The Coronavirus Pandemic boosts adoption of remote microsampling devices and kits for COVID-19 studies by the National Institutes of Health (NIH) and other research centers that allow people to self-sample at home. Virtual clinical trials and remote patient monitoring improve the patient experience. Thousands of Mitra kits are deployed globally, including new Pediatric Collection Kits tailored for researchers seeking a gentler, safer sampling solution for children.
Neoteryx is acquired by Trajan.
Trajan Scientific and Medical recognizes the promise of microsampling to benefit more people and acquires Neoteryx to expand its microsampling product portfolio. They offer Mitra® with VAMS®, hemaPEN®, Harpera®, and other devices and tools for a range of needs. Trajan moves closer to realizing its vision to enrich personal health through scientific tools and solutions.

what's next...
The microsampling brand of Trajan, Neoteryx supports microsampling across industries around the globe. Our customers choose from multiple microsampling solutions to suit different project needs.
sample small. think big.
The possibilities for microsampling innovations & collaborations are endless. Have a question or idea? We invite you, the adopters, innovators and thought leaders in microsampling, to reach out to us!
explore our gold-standard microsampling technologies, industry resources and technical information.









our latest news

Mitra® VAMS® Microsampling Collection Video Now Available in Spanish and Arabic

Success with Microsampling in Clinical Trials Live Discussion

Trajan appoints AppMed Inc. as Canadian distributor for Microsampling technologies, featuring Mitra® and Harpera®
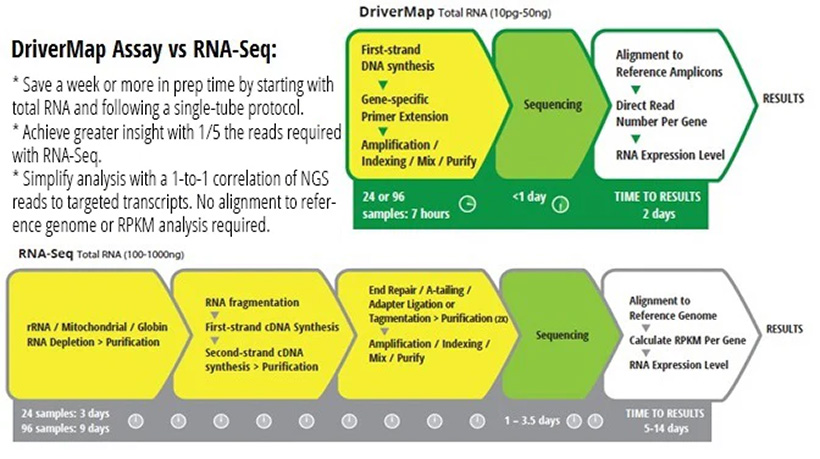
Neoteryx and Cellecta partner to introduce innovative finger-prick blood test for comprehensive whole-genome expression profiling
Neoteryx EU authorized representative update

