Safer-at-Home, Simple, Precise
No matter who or where you are, we offer a complimentary, virtual meeting with one of our Microsampling Consultants to help you select the best microsampling device for your needs.
Those participating in studies can easily collect their own biological specimen at home. This eliminates inconvenient facility visits and enables modern, precise study designs.
Gain access to large or underserved populations of research subjects. Improve recruitment by offering a convenient way to provide specimens, even in low-resource regions.
Go virtual! Clinical trial subjects don't need to be onsite to participate. With Mitra remote collection devices, they collect their specimens at home as often as needed for better scientific insights.
Our future depends on smarter, safer healthcare. You can use Mitra® Microsamplers from Neoteryx to conduct the groundbreaking research and decentralized clinical trials that will fuel the future.
High sample quality, precise volume
(< 4% RSD) collection
Simply touch the Mitra® device tip to a bio fluid and watch it absorb in seconds
Transport specimens without cold-chain shipping. Eliminate freezers for storage
Send Mitra samples through the mail and process them in a 96-well format
140+ Mitra research papers illustrate the utility of VAMS® technology
Volumetric collection enables precise & quantitative data to be generated
Mitra blood microsampling offers benefits throughout the drug development pipeline with both large and small molecules - from facilitating serial sampling in animal models for preclinical drug research to offering improved convenience and access in Phase 3 human trials.
Remote Mitra sampling broadens the subject recruitment pool for large population studies, while its convenience also improves subject compliance & retention.
Remote Mitra sampling enables seroprevalence studies of viral pathogens and insight into associated biomarkers among individuals who remain safely at home during infectious disease outbreaks & social distancing restrictions.
Novel studies of newborns and children is possible with microliter samples that are low-stress and convenient for parents and others to facilitate.
Mitra samples are precise, allowing researchers to accurately capture, analyze genome, proteome, metabolome, and microbiome data from a few drops of biological fluid.
Remote sampling can unlock the potential of precision medicine by enabling longitudinal studies using remote samples to monitor biomarkers in diverse study populations.
Don't worry, Mitra devices are used for a wide range of research applications. Tell us how you want to use microsampling and we will help you determine if Mitra is a good fit!
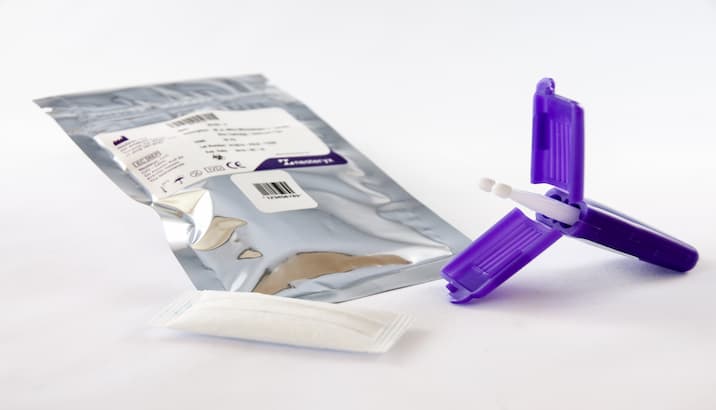
This easy-to-use format has two sampling devices, includes barcoding, and is enclosed in a specimen bag for convenient transport.
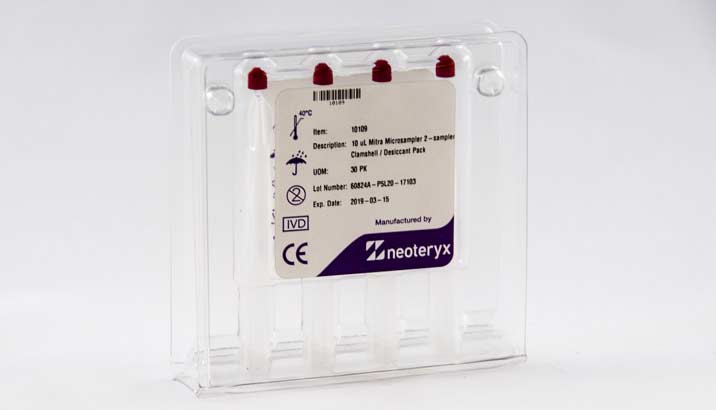
This budget-friendly option is available with 2 or 4 samplers inside and works well for pilot studies, proof-of-concept testing, and clinical research with limited funding.
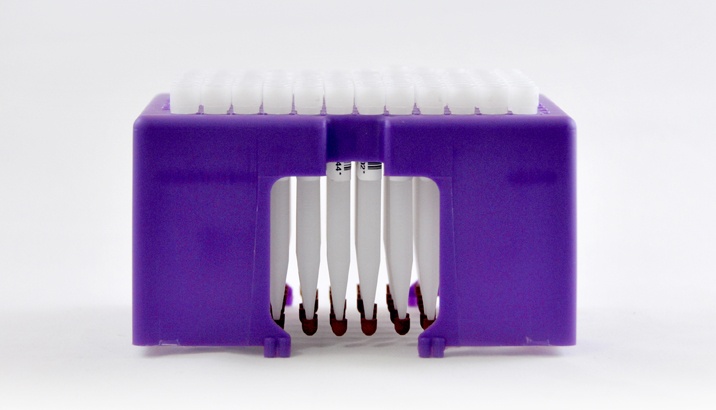
The 96-autorack is compatible with standard 96-well plates and facilitates high throughput sample processing.
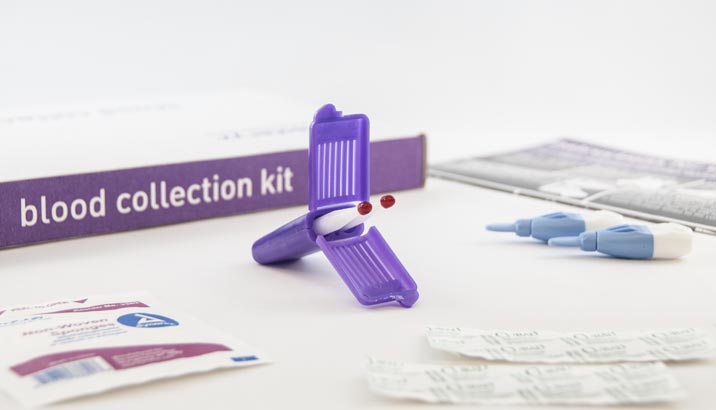
The Clamshell and Cartridge Mitra formats are available packaged in specimen collection kits. Kits can be customized to your needs.
Neoteryx created the Mitra® Microsampler with you in mind.
Whether you are a clinical research participant, CRO lab director, research scientist, clinical trial manager or technician, there is a Mitra Microsampler that will work for you. We'll help you select the device format that fits you best via a virtual demonstration with a Microsampling Consultant.









.png)



Contact Us
Trajan Scientific Americas Inc.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.
Mitra® devices are CE-IVD (IVDR) devices intended as specimen collectors and for the storage and transport of dried blood. They are available as registered IVD Devices in the European Union and United Kingdom, Australia, Brazil, China, and Canada, as well as multiple Health Ministries worldwide. In the USA, Mitra devices are supplied as a research use only (RUO) product to assist in method development, other research-related and non-diagnostic activities. Mitra has not been validated for use with any diagnostic testing.
hemaPEN® variants are CE-IVD (IVDR) devices intended as specimen collectors, for the storage and transport of dried blood specimens and are available as registered IVD Devices in the European Union and United Kingdom, Australia/New Zealand, and the USA. Outside of these territories, the hemaPEN is supplied for research use only (RUO).
The Harpera™ skin microbiopsy tool is currently supplied as an investigational use only (IUO) product globally.