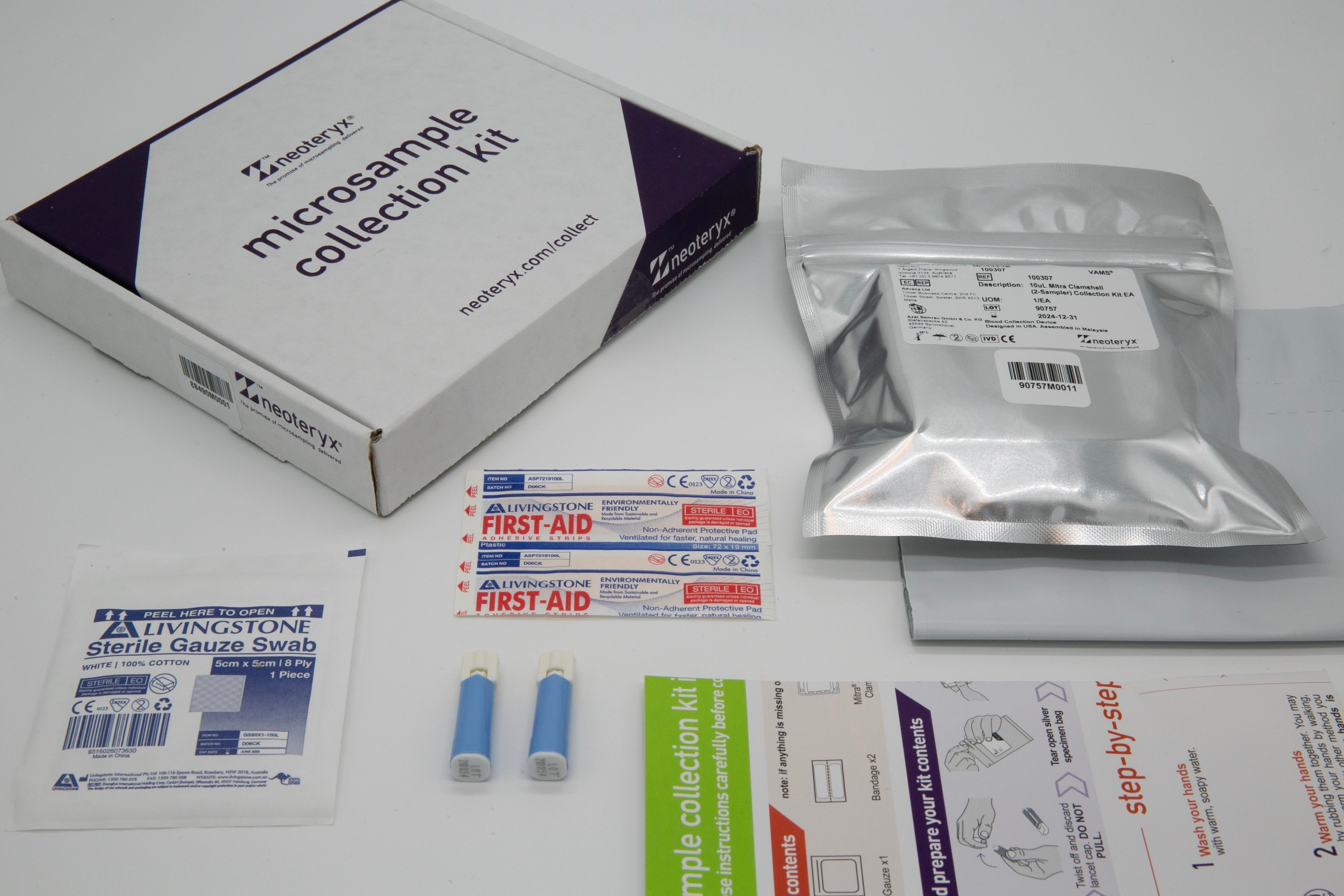
microsampling product advantages
Our remote specimen collection devices enable accurate microsampling in science and medicine.

microsampling can replace some unnecessary needle draws...
Consider offering portable devices for convenient sampling anywhere.

microsampling can improve the specimen collection experience...
People can send their specimen samples via the nearest mailbox.

microsampling can fit into the lab workflow...
Dried microsamples interface with commonly used methods & lab instruments.

microsampling can support precision analysis...
Studies show that dried matrix microsamples deliver dependable data.

microsampling can cut costs & boost enrollment...
Decentralized trials & remote sampling can increase recruitment & reduce costs.
our microsampling products

Harpera | Skin Microbiopsy
call us for microsampling support
Our technical director & microsampling specialists are available to answer questions and ensure your project's success.

contact us to get started!
Are you ready to start microsampling? Let's begin with a customer consult. schedule a meeting